Meeting Summary
Cholangiocarcinoma (CCA) is a cancer of the bile duct with poor prognosis and increasing incidence. Filippo de Braud gave an overview of CCA and its classification and highlighted key unmet needs in both diagnosis and treatment. Juan Valle explained that intrahepatic CCA (iCCA) accounts for 11% of the entire cancer of unknown primary (CUP) population, as well as 33% of patients with liver-inclusive disease. It is, therefore, vital that CUP with a ‘liver-dominant lesion’ is reviewed by a specialist team familiar with iCCA management. Angela Lamarca emphasised that managing CAA requires a multidisciplinary approach to both diagnosis and treatment. Precision medicine is now a reality in biliary tract carcinoma (BTC), particularly iCCA, so molecular testing is essential to ensure targeted therapy options are considered. David Malka explored existing and emerging standard-of-care (SOC) treatments for advanced BTC, which represents a target-rich disease. Updated guidelines now recommend targeted treatment for second-line iCCA in patients with appropriate alterations, underscoring the importance of systematic and early molecular profiling. A wide range of malignancies harbour FGFR alterations at varying frequencies, and the FGFR inhibitor pemigatinib is already approved for the treatment of CCA with FGFR2 fusions or rearrangements. Antoine Hollebecque highlighted the final results from the Phase II FIGHT-202 study, in which pemigatinib achieved an overall response rate (ORR) of 37% in the target population with a manageable safety profile.
Cholangiocarcinoma: The Little-Known, Rare Cancer of the Biliary Tract
Filippo de Braud
CCA is a blanket term encompassing three diseases (intrahepatic, perihilar, and distal CCA), which vary according to their anatomical site.1 Extrahepatic CCA (eCCA) includes both perihilar and distal CCA.1 CCA is a rare cancer, currently accounting for approximately 3% of all gastrointestinal tumours and 10–25% of all primary hepatobiliary cancers.2,3 The global incidence is variable but increasing, de Braud explained, with observed increases likely driven partly by lifestyle and metabolic-related factors.4 Incidence of iCCA appears to be increasing at a faster rate than eCCA.4 Currently, the prognosis for patients with CCA remains poor, with less than 20% surviving for 5 years after diagnosis.1
Although most cases of CCA arise without apparent cause, some established risk factors do exist and exert a direct influence on the intra- or extrahepatic aetiology of CAA.5,6 De Braud outlined how these risk factors can be considered to be related to benign liver disease (e.g., bile duct stones), inflammatory status (e.g., primary sclerosing cholangitis), or infection (e.g., liver fluke).7 Metabolic-associated conditions may also impact disease epidemiology based on recent evidence from over 2,000 European patients with CCA, which revealed that 55.1% were overweight/obese and nearly one-quarter (22.5%) had diabetes.8
The heterogeneity of CCA in primary tumour location creates key diagnostic challenges, compounded by the fact that the disease is often advanced by the time patients become symptomatic. De Braud explained that iCCA, in particular, is associated with non-specific symptoms such as abdominal pain and malaise, leading to incidental diagnosis in 20–25% of cases.2,5,9,10 Although eCCA provokes painless jaundice in 90% of patients, acute cholangitis (found in 10% of patients) can sometimes lead to misdiagnosis of benign disease.9 Numerous therapeutic challenges also exist in CCA, including the different surgical approaches and potential complications, the feasibility of obtaining biopsy tissue for molecular profiling, and the heterogenous response to treatment.11 Together, these challenges demand a co-operative multidisciplinary team (MDT) approach to CCA management.
CCA is also molecularly heterogenous, with molecular patterns changing, according to the primary tumour location and aetiology.12,13 IDH1 mutations and FGFR2 fusions are two of the most commonly occurring gene alterations detected in iCCA.12 Molecular characterisation of patients with CCA should, therefore, be mandatory, de Braud insisted, because targeted therapy can help to improve clinical outcomes of the disease.
Survival rates achievable with currently used systemic treatment options highlight the clear unmet need in CCA. In the pivotal ABC-02 study, the combination of cisplatin and gemcitabine (gem/cis) performed better than single-agent gemcitabine, with a median overall survival (OS) of 11.7 months.14 With the addition of durvalumab immunotherapy to gem/cis, median OS remained at approximately 1 year, although the 2-year survival rate more than doubled (24.9% versus 10.4%).15 Second-line treatment is also associated with minimal benefit over active symptom control for molecularly unselected patients with CCA, as demonstrated in the ABC-06 study.16 Second-line treatment with a modified folinic acid plus fluorouracil plus oxaliplatin regimen (mFOLFOX) produced some improvement in OS but was associated with increased Grade ≥3 adverse events.16
In summary, de Braud reiterated the important diagnostic and therapeutic challenges faced in CAA. Patients are generally asymptomatic in the early stages, with symptoms only appearing at more advanced disease stages when prognosis is worse; misdiagnosis can also occur. Only approximately 30% of patients are suitable for surgical resection, which currently represents the only potentially curative option for CAA.1,5,17 The vast majority of patients (approximately 70%) remain inoperable and derive little benefit from first-line chemotherapy, with only modest response rates and poor OS.1,5,17 Collectively, these limitations in current treatment options underscore the need for molecular profiling at diagnosis to pinpoint those patients suitable for therapy with molecularly targeted agents. This is where the future lies for the treatment of CAA, de Braud concluded.
The Challenges Associated with Diagnosing Intrahepatic Cholangiocarcinoma for Targeted Therapies
Juan Valle
Symptoms of iCCA are non-specific, so late presentation is common, and challenges in the differential diagnosis are compounded by non-definitive radiological assessment and non-specific histopathology.18 Even in ‘expert hands’, as many as 14% of patients with non-hepatocellular carcinoma (HCC) may be incorrectly designated as HCC based on radiological assessment, noted Valle.19 Pathology hinges on the clinical details provided to the pathologist, who usually does not have sight of the imaging.20 However, there is no tissue that definitively identifies CCA, and the immunohistochemistry profile overlaps considerably with metastatic adenocarcinoma.21 Valle stressed that the hepatopancreatobiliary MDT assessment is, therefore, key in order to consider the totality of the evidence and reach a diagnosis.22
Another avenue by which patients present is the CUP route, which accounts for 3–5% of all cancer diagnoses.23 Confirmed CUP requires a metastatic epithelial or neuroendocrine malignancy on final histology with no primary site detected, despite an initial screen of investigations, specialist review, and further specialised investigations as appropriate.24 According to European Society for Medical Oncology (ESMO) guidelines, patients may fall within a specific subset of CUP that determines specific treatment; however, currently, there is no pattern suggestive of iCCA.23
The use of molecular profiling to predict tissue of origin in CUP has previously shown that most patients have primary tumours in the biliary tract.25 Against this backdrop, a recent retrospective study was conducted to identify iCCA ‘hidden’ within the CUP population. Case notes from 228 sequential patients between January 2017 and April 2020 underwent radiology review.26,27 Results revealed that 32% of patients with confirmed cancer of an unknown primary had malignancy involving the liver, and one in three showed radiological features of iCCA.26 Overall, a potential iCCA diagnosis was identified in 11% of the entire group, which is equivalent to one in nine patients with CUP, Valle emphasised. According to Conway et al.,26 of these patients with potential iCCA, 63% had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, making them fit enough to consider treatment. In general, patients with iCCA identified in this study tended to be younger (mean age: 63 years), with a 5-year age gap versus the entire CUP group (mean age: 68 years) and were predominantly (75%) female.26 Notably, two-thirds (62.5%) had not been discussed at a previous hepatopancreatobiliary MDT. Valle noted that, although tissue was not available for all patients with iCCA within the CUP population, the mutational analysis revealed the ‘usual drivers’, including molecular alterations in IDH1, FGFR, and BRAF genes.26
Based on this evidence, Valle proposed a revision to the pathway for CUP moving forward, with iCAA considered as one of the specific subsets.26 So, if you see adenocarcinoma with a dominant liver lesion(s), think about iCCA, Valle stressed. Improved identification of iCCA would then enable molecular profiling for targetable alterations to be performed and for patients to receive specific treatment. To this end, the CUPISCO study28 is an important Phase II trial that aims to determine the efficacy and safety of targeted therapies and immunotherapy for patients with a subset of CUP. After screening, CUP patients undergo genomic profiling of tissue and blood before treatment with three cycles of induction chemotherapy. Patients who achieve disease control are then randomised 3:1 to additional chemotherapy or choice from a ‘menu’ of targeted therapies or immunotherapy options based on their mutation profile. Valle explained that the CUPISCO is still recruiting, with a planned enrolment of 790 patients, but has already revealed some important lessons.29 In 124 patients (35.8%), the diagnosis of unfavourable adenocarcinoma or poorly differentiated CUP could not be confirmed.30 Of the eligible patients, seven (5.7%) were found to have iCCA.30 As part of the CUPISCO study, an eligibility review algorithm has been developed for diagnostic workup and identification of iCCA.30 Key steps include obtaining advice from a reference radiologist as part of the MDT review.
Summarising the take-home messages, Valle acknowledged that most liver lesions are not CCA and may be a primary tumour such as HCC or secondary metastatic, making biopsy essential. Nevertheless, CCA must be considered, Valle stressed, as iCCA accounts for 11% of the entire CUP population and one-third of patients with liver-inclusive disease.26 Valle concluded that CUP with a ‘liver-dominant’ lesion should, therefore, be reviewed by a specialist team familiar with iCCA management.
Optimising Molecular Profiling and the Multidisciplinary Team Approach in Cholangiocarcinoma
Angela Lamarca
The landscape of CCA management has become more complex over recent years, with an increasing number of treatment options available and under development.1 Therefore, adopting an MDT approach is central to CCA management in 2022, Lamarca reiterated, to overcome established and emerging diagnostic and treatment challenges. Currently, there are no standardised guidelines for MDTs in CCA, although the recent ENS-CCA survey has provided some recommendations.31 These recommendations include the required presence of an MDT co-ordinator, weekly MDT meetings, the need for collective discussions, and the presence of mandatory specialists.31
The multidisciplinary approach will become ‘even more ‘relevant’ as we enter the era of molecular profiling and precision medicine for patients with CCA, Lamarca explained. Radiation oncologists, interventional radiologists, pathologists, and molecular pathology scientists, in particular, have an essential role in therapeutic decision-making. For targeted therapies, molecular profiling is key to pinpointing those patients with iCCA with targetable alterations who stand to benefit from this novel treatment approach.
Biliary tract tumours are heterogenous at a molecular level, and alterations found in iCCA differ from those in eCCA and gallbladder cancer.12 Lamarca commented that, in an ‘ideal world’, molecular profiling would be carried out on every patient with CCA with the goal of deploying targeted treatment options where appropriate; however, there are hurdles to delivering this in real-world clinical practice. Key challenges associated with molecular profiling lie in the lack of quality tissue and the high rate of failed analysis. In an example from their centre, Lamarca outlined how targetable alterations had been detected in 40% of patients, but one in four tissue samples had failed analysis due to insufficient tumour content.32 To overcome this, Lamarca advocated working with the pathologist to select the best blocks and considering early re-biopsy if there is insufficient tissue for analysis.
On the subject of when and how to test patients, Lamarca stressed that molecular profiling should be carried out as soon as possible following diagnosis, especially in patients with advanced disease. Molecular testing is time-consuming, Lamarca explained, “so the sooner that the process is started, the sooner we get results and the better we can plan future steps.” Unless patients are enrolled in a clinical trial, it is not recommended to withhold first-line therapy while awaiting results. Both multigene tumour next-generation sequencing (NGS) and fluorescence in situ hybridisation are suitable molecular testing methods for patients with CCA.33,34 Lamarca advised choosing a wide gene panel, encompassing not only FGFR but also other targetable alterations. RNA-based NGS may provide better sensitivity for detecting gene fusions and circulating tumour DNA may be useful if tissue is unavailable and/or a new biopsy is not feasible. Current recommendations from the ESMO Precision Medicine Working Group list IDH1, FGFR2, and NTRK genomic alterations as top priorities for testing in advanced CCA and echo the importance of RNA-based assays for the identification of fusion transcripts, as in FGFR.33,34
Overall, Lamarca concluded that an MDT approach to CCA management is required, not just in diagnosis but for treatment decision-making around locoregional strategies and targeted therapies. Precision medicine is now a reality in biliary tract cancer, especially for iCCA, where targetable alterations of relevance include FGFR2 fusions, IDH1 mutations, and others. To bring precision medicine into the clinic, NGS or fluorescence in situ hybridisation testing is needed early in the patient’s journey, making adequate tissue or liquid biopsy essential.
Clinical Practice Guidelines for Biliary Tract Cancer: Updates for the Management of Patients with Locally Advanced or Metastatic Intrahepatic Cholangiocarcinoma
David Malka
Malka described how the management of BTC has progressed significantly over the last decade, with developments in chemotherapy, immunotherapy, and targeted treatments. The year 2022 represents a particularly important time for clinical practice guidelines in BTC, with updates from the National Comprehensive Cancer Network (NCCN) in the USA, as well as ESMO and the French group, Le Thésaurus National de Cancérologie Digestive (TNCD), in Europe.22,35,36
Overall, Malka explained that the last decade has yielded three critical learnings in the field of BTC. First has been the establishment of SOC chemotherapy with the introduction of gem/cis in 2010. This regimen is now recommended first-line in both USA and TNCD guidelines.35,36 FOLFOX is the NCCN’s preferred regimen for subsequent-line therapy and is also recommended second-line in TNCD guidelines in the absence of the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) I–II target alterations.35,36
The second key learning of the last decade is that BTC, especially iCCA, is a target-rich disease that warrants early molecular tumour profiling during (or even before) first-line treatment. Developments in targeted therapies began with preclinical studies underlining the importance of FGFR2 and IDH alterations in iCCA, explained Malka, and has led to an ‘ever-growing constellation of gene inhibitors’ with U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval. For both first-line and subsequent therapy, NCCN guidelines list specific gene-targeted therapies as useful in certain circumstances for patients with actionable alterations.35 The French TNCD guidelines recommend that a tumour molecular portrait be carried out systematically within first-line (or even before referral to clinical trials). This should include mismatch repair status, HER2 expression, as well as NGS (by DNA or RNA analysis) searching for targetable tumour mutations and NGS (by RNA analysis) to look for fusions/rearrangements, including FGFR2 and NTRK genes.36 Targeted agents recommended as second-line treatment and beyond with an ESCAT I–II level of evidence for patients with appropriate alterations include: pemigatinib, futibatinib, and infigratinib for FGFR2 fusion; zanidatamab for HER2 overexpression; adagrasib for KRAS mutations; and larotrectinib for NTRK fusions.36
The third and final key learning point is that, in addition to precision medicine, BTC tumours also benefit from immunotherapy, which Malka described as “the second therapeutic revolution in 21st century oncology.” The efficacy of adding durvalumab to gem/cis was demonstrated in the TOPAZ-1 trial, and this combination is now recognised in NCCN guidelines as a Category 1 preferred regimen for primary treatment.35 French guidelines also recommend durvalumab-gem-cis if available, for first-line therapy.36
Malka highlighted some important questions and challenges that remain in BTC management. Key issues to be addressed in molecular profiling include the optimal management of samples, affordability, timing, and the adoption of a one-size-fits-all versus a subtype-specific approach (i.e., iCCA versus perihilar/distal CCA versus gallbladder cancer). It will also be essential to understand the optimal therapeutic positioning of targeted agents, whether will they move to first-line treatment, and explore issues around resistance mechanisms, therapeutic triage, and sequencing, particularly regarding FGFR2 inhibitors. One study attempting to answer some of these questions is the ongoing SAFIR ABC10 trial. In this study, patients with advanced BTC will start first-line SOC and undergo tissue and liquid molecular profiling. Those with targetable alterations and disease control after 12 weeks will then undergo 2:1 randomisation to matching targeted therapy versus continuation of SOC. A further important consideration is the exportability of clinical trial data to the real-world perspective, noted Malka. A recent analysis of 3,650 iCCA cases from a French nationwide hospitalisation database revealed that the vast majority of patients (65%) were managed with the best supportive care only and over 70% were treated in low/intermediate volume centres with minimal iCCA experience.37
Summing up, Malka highlighted that the last decade in BTC has seen the emergence of SOC across all disease settings, with the immunochemotherapy triplet regimen of durvalumab-gem-cis potentially becoming the new first-line SOC.18,35,36 BTC, especially iCCA, is a target-rich disease, underscoring the importance of a systemic policy of early molecular profiling within or before the first line.38 The rarity and heterogeneity of BTC, coupled with the complexity of therapeutic management, also argues for patient referral to high-volume centres where possible, Malka concluded.
Targeting FGFR Alterations in Cholangiocarcinoma and Other Solid Tumours
Antoine Hollebecque
FGF/FGFR signalling plays a central role in multiple cellular processes, and the deregulation of this signalling pathway is implicated in tumourigenesis.39.40 Specifically, aberrant FGFR signalling mediates tumourigenesis by enhancing cellular proliferation, migration, survival, invasion, and angiogenesis.39,40 Hollebecque explained that three different genomic alterations might result in tumourigenic FGFR signalling: activating mutations, chromosomal rearrangements, and gene amplification.40
A wide range of malignancies carry FGFR alterations at varying frequencies.41-43 In iCCA, the estimated frequency of FGFR2 fusions ranges from 10–20%.41-43 Several FGFR inhibitors are already licensed in the oncology arena, including pemigatinib, which is approved for the treatment of CCA with FGFR2 fusions or rearrangements in both Europe and the USA, and infigratinib, which has FDA approval for the same indication.44-46 The FGFR inhibitor erdafitinib is also approved in the USA for locally advanced or metastatic bladder cancer with susceptible FGFR2/3 genetic alterations.47
The efficacy of pemigatinib in CCA with FGFR2 fusions or rearrangements was demonstrated in the FIGHT-202 study, the final results of which were presented.48 FIGHT-202 was a Phase II, open-label, single-arm study involving 147 patients previously treated with locally advanced or metastatic CCA. The study included three cohorts: Cohort A (n=108) with FGFR2 fusions or rearrangements; Cohort B (n=20) with other FGF/FGFR genetic alterations; and a control cohort, Cohort C (n=17), with no FGF/FGFR genetic alterations. Patients received oral pemigatinib at a dose of 13.5 mg once daily for 2 weeks followed by 1 week off therapy until disease progression or toxicity. The primary endpoint was ORR in Cohort A as confirmed by an independent central review. Secondary endpoints included ORR in other cohorts, duration of response, progression-free survival (PFS), OS, and safety. Outlining demographics and baseline clinical characteristics for the FIGHT-202 study, Hollebecque explained that Cohort A included a greater proportion of females than the other two cohorts and that 40% of patients were heavily pre-treated, having received two or more prior systemic therapies.48
Pemigatinib achieved an ORR of 37% in Cohort A, while no responses were observed in Cohorts B or C (Table 1). Disease control rate was also higher in cohort A (82%) as compared with both Cohort B (40%) and Cohort C (18%). Median duration of response was 9.1 months among the 40 patients in Cohort A who responded to pemigatinib. Among 104 evaluable patients, the median best percentage change from baseline in the sum of target lesion diameters was −28.4% (range: −100%–+55%).48
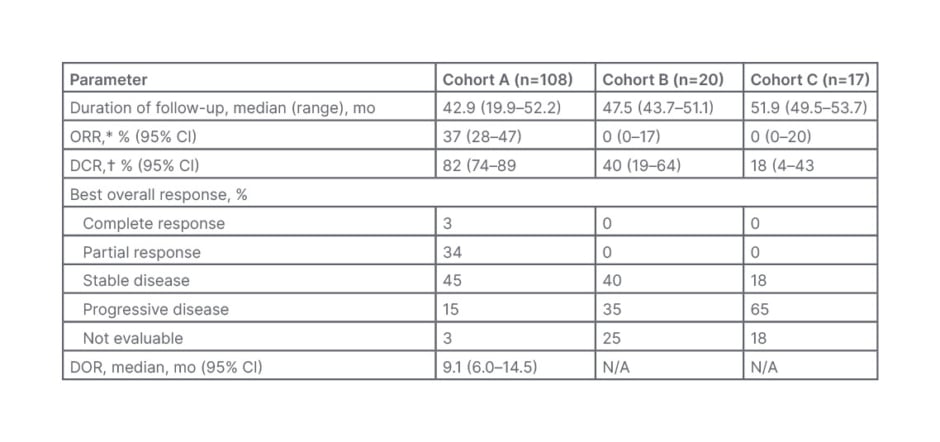
Table 1: Response to pemigatinib in the FIGHT-202 study.48
CI: confidence interval; DCR: disease control rate; DOR: duration of response; mo: months; N/A: not applicable; ORR: overall response rate.
*ORR is complete response+partial response.
†DCR is complete response+partial response+stable disease.
Reproduced with permission from Vogel et al.48
OS and PFS results confirmed the benefit of pemigatinib in CCA with FGFR2 fusions or rearrangements. (Figure 1) Median PFS was 7.0 months in Cohort A compared with 2.1 months and 1.5 months in Cohorts B and C, respectively. Median OS was also substantially longer in Cohort A (17.5 months) versus Cohort B (6.7 months) and Cohort C (4.0 months).48
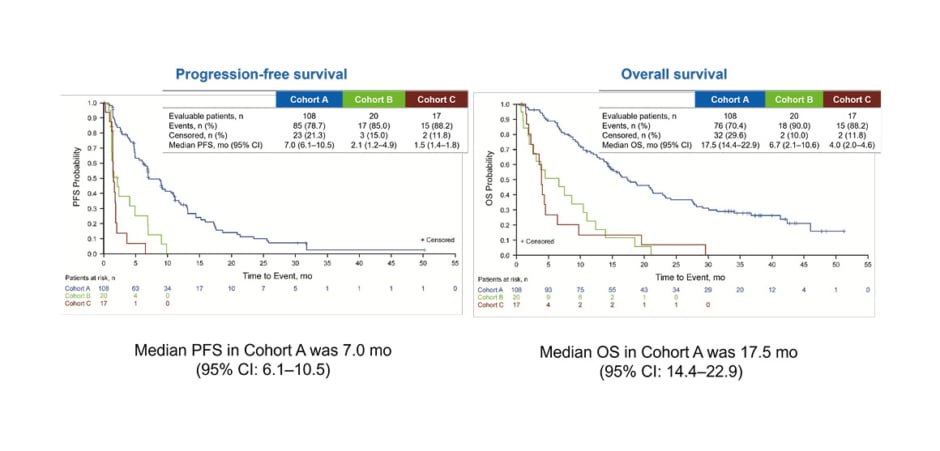
Figure 1: Progression-free survival and overall survival in the FIGHT-202 study of pemigatinib.48
CI: confidence interval; mo: months; OS: overall survival; PFS: progression-free survival.
Reproduced with permission from Vogel et al.48
Pemigatinib showed a manageable safety profile consistent with the primary publication, with no new safety signals observed.48,49 Hollebecque pointed out that most treatment-emergent adverse events were low-grade and related to FGFR inhibition. Hyperphosphataemia was the most common treatment-emergent adverse events in 59% of pemigatinib-treated patients, with no cases Grade ≥3.48
In addition to pemigatinib, other FGFR inhibitors have also demonstrated efficacy in this FGFR2 rearrangement–positive CCA population.48,50-52 Without head-to-head studies, cross-study comparisons of efficacy or safety cannot be made, but, in general, ORRs ranged from approximately 20–40%, with a median PFS of 7‒8 months.48,50-52 Median OS was also longer in patients treated with FGFR inhibitors compared with what we know for all-comers with CCA, Hollebecque added, ranging from approximately 12–20 months.48,50-52
Hollebecque went on to present results from clinical studies of FGFR inhibitors in other solid tumours. The benefit of FGFR inhibition was demonstrated in the BLC2001 study of erdafitinib in patients with urothelial carcinoma, with ORR (40%), PFS (5.5 months), and OS (11.3 months) rates comparing favourably with historical results.53,54 Higher response rates were achieved in patients with FGFR3 mutations as compared with FGFR2/3 fusions.53,54 Similarly, the FGFR2b-targeted monoclonal antibody bemarituzumab met both primary (PFS) and secondary (OS) endpoints in a study involving patients with gastric cancer.55 In this placebo-controlled study, bemaritzumab plus mFOLFOX was compared with mFOLFOX alone.55
Trials have also evaluated FGFR inhibition in agnostic tumours. The FIGHT-101 study assessed pemigatinib (at various doses from 1–20 mg once daily intermittently [2 weeks on/1 week off] or continuously) in 128 patients with refractory advanced malignancies with and without FGF and FGFR gene alterations. ORR was highest for patients with FGFR fusions or rearrangements (25.0%), followed by those with FGFR mutations (23.1%), whereas patients with FGF or FGFR amplifications did not respond as well (5.0% or 3.8%).56 In the pivotal Phase II study, RAGNAR, erdafitinib showed an ORR of 26.4% in the tumour-agnostic population, with 66.3% of patients experiencing a reduction in tumour burden.57
Summing up, Hollebecque explained that different genomic alterations, fusions/rearrangements, mutations, and amplifications, may result in tumourigenic FGFR signalling. FGFR inhibitors have demonstrated antitumour activity in various solid tumours harbouring these FGFR alterations, and a number of FGFR inhibitors are already approved for treatment, including pemigatinib for CCA with FGFR2 fusion or rearrangements. Moving forward, additional data are required to define the best target population and FGFR alteration, Hollebecque concluded.
EU/PEMA/NP/22/0030
Date of preparation: December 2022








