Authors: George W. Sledge Jr.,1 Sara M. Tolaney,2 Zefei Jiang3
1. Medical Oncology, Stanford University School of Medicine, Stanford, California, USA
2. Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA
3. Department of Breast Oncology, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, China
Disclosure: Prof Sledge has received clinical trial support, research grants, and travel/accommodation/expenses from Eli Lilly and Company; research grants from Pfizer; is on the board of directors for Tessa Therapeutics; and has been a consultant for Syndax, Symphogen, and Verseau Therapeutics. Dr Tolaney has received institutional research support and has served as a consultant/advisor for Merck, Bristol-Meyer Squibb, Eli Lilly and Company, Pfizer, Novartis, AstraZeneca, Eisai, Nektar, Sanofi, Immunomedics, and Genentech; received further research support from Exelixis, Odenate, and Cyclacel; and has been a consultant/advisor for Celldex, Paxman, Seattle Genetics, Nanostring, Daiichi-Sankyo, Athenex, and Puma. Dr Jiang has declared no conflicts of interest.
Acknowledgements: Medical writing assistance was provided by Dr Brigitte Scott, MarYas Editorial Services, Cowlinge, UK.
Support: This article was funded by an educational grant from Eli Lilly and Company and includes data presented at the European Society for Medical Oncology (ESMO) Congress held on 28–29th September 2019 in Barcelona, Spain. It may include opinions of speakers that do not necessarily reflect those of Eli Lilly and Company.
Citation: EMJ Oncol. 2020;8[Suppl 2]:2-9.
Meeting Summary
Most patients with metastatic breast cancer have hormone receptor-positive (HR+) tumours and are initially treated with endocrine therapy (ET).1-4 Cyclin-dependent kinase (CDK) 4/6 inhibitors in combination with ET are now considered a standard-of-care treatment for patients with HR+, human epidermal growth factor receptor 2 (HER2)-negative (-) advanced breast cancer (ABC).5,6 Abemaciclib is a selective CDK4/6 inhibitor (14-times more potent against CDK4 than CDK6 in enzymatic assays)7 and is administered orally, twice daily on a continuous schedule.1 Abemaciclib is the only CDK4/6 inhibitor approved for monotherapy after progression on ET and prior chemotherapy in the metastatic setting in the USA (MONARCH 1).7,8 This inhibitor is also approved in combination with ET in an initial setting with an aromatase inhibitor (MONARCH 3) and after progression on ET with fulvestrant (MONARCH 2).1,8-11
This article summarises the data from three poster presentations (late-breaking abstracts) that took place on 28–29th September 2019 as part of the European Society for Medical Oncology (ESMO) Congress in Barcelona, Spain. Discussed are the overall survival (OS) results from the prespecified interim analysis of the Phase III study, MONARCH 2, in patients with HR+, HER2- ABC who progressed on ET and received abemaciclib or placebo with fulvestrant. Further discussed is progression-free survival (PFS) interim data from MONARCHplus, a Phase III study that evaluated abemaciclib plus ET in predominantly Chinese patients with HR+, HER2- ABC; and results from monarcHER, a Phase II study of abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in patients with HR+, HER2-positive (+) ABC.
Abemaciclib plus Fulvestrant Improves Overall Survival in Women with ET-Resistant HR+, HER2- Advanced Breast Cancer (MONARCH 2)
Professor George W. Sledge Jr
MONARCH 2 was a global, randomised, double-blind, Phase III study of abemaciclib or placebo in combination with fulvestrant in premenopausal or perimenopausal women (with ovarian suppression) and postmenopausal women with HR+, HER2- ABC that progressed during prior ET.1,10,12 Sledge et al.11 randomised 669 patients (2:1) to receive abemaciclib 150 mg every 12 hours (q12h) or placebo on a continuous schedule, plus fulvestrant 500 mg administered per label. Randomisation was stratified based on the site of metastasis (visceral, bone-only, or other) and resistance to prior ET (primary versus secondary).13
In 2017, the authors reported that abemaciclib plus fulvestrant compared with placebo plus fulvestrant significantly improved investigator-assessed PFS (primary endpoint) (median: 16.4 versus 9.3 months; hazard ratio [HR]: 0.553; 95% confidence interval [CI]: 0.449–0.681; p<0.001) and objective response rate (ORR) (measurable disease: 48.1% versus 21.3%; p<0.001) with a generally tolerable safety profile.1 At the time of primary PFS reporting,11 the data for OS (an important secondary endpoint in the study) were immature, so presented here are updated PFS data, OS results, and time to chemotherapy (exploratory endpoint) in the intent-to-treat (ITT) population of the prespecified interim analysis of MONARCH 2 at approximately 77% maturity (338 deaths of the planned 441).10
At the time of interim analysis (median follow-up of 47.7 months), 17.3% of patients in the abemaciclib arm remained on treatment versus 3.6% on the placebo arm. Updated PFS results were highly consistent with those of the primary analysis: median of 16.9 versus 9.3 months for the abemaciclib versus placebo arms (HR: 0.536; 95% CI: 0.445–0.645; p<0.0001).11 The authors reported that the median OS with abemaciclib plus fulvestrant was 46.7 compared with 37.3 months with placebo plus fulvestrant; this 9.4-month OS benefit was statistically significant (HR: 0.757; 95% CI: 0.606–0.945; p=0.0137 [hl]Figure 1[/hl]).10,11
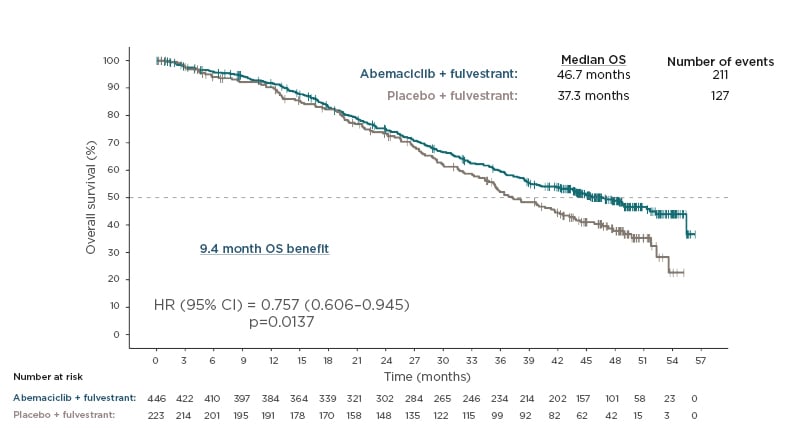
Figure 1: Overall survival.
Overall survival in hormone receptor-positive, human epidermal growth factor receptor 2 advanced breast cancer in MONARCH 2.10
HR: hazard ratio; OS: overall survival; 95% CI: 95% confidence interval.
A closer look at OS according to the prespecified stratification factors (nature of disease and ET resistance) revealed no statistically significant differences, as shown by the interaction p values (0.424 and 0.588, respectively [hl]Figure 2[/hl]).10,13 The authors highlighted the more pronounced effects in patients with visceral disease who appeared to derive a clear benefit (median OS: 40.3 versus 32.2 months for abemaciclib versus placebo arms; HR: 0.675; 95% CI: 0.511–0.891) and primary resistance to prior ET (median OS: 38.7 versus 31.5 months for abemaciclib versus placebo arms; HR: 0.686; 95% CI 0.451–1.043).11 The 95% CI for primary resistance to prior ET crossed 1, which shows there is insufficient evidence to conclude that the groups are statistically significantly different. Addition of abemaciclib to fulvestrant also significantly prolonged time to chemotherapy from 22.1 to 50.2 months in the placebo and abemaciclib arms, respectively (HR: 0.625; 95% CI: 0.501–0.779; p<0.0001).11 Safety data were consistent with that of the primary analysis, with diarrhoea, neutropenia, and nausea being the most common adverse events (AE).10,11 The authors concluded that the addition of abemaciclib to fulvestrant provided a statistically significant OS improvement in patients with HR+, HER2- ABC who progressed on prior ET.10
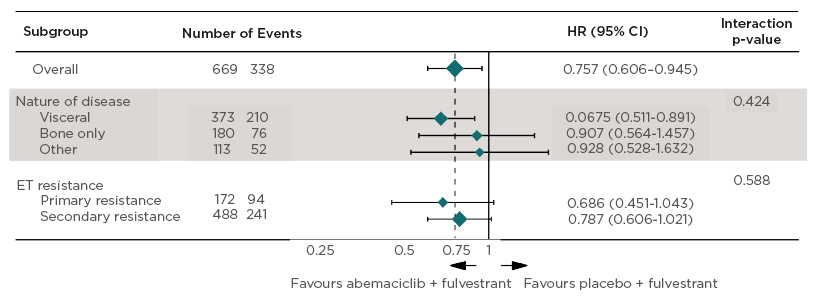
Figure 2: Overall survival by stratification factors.
Overall survival by stratification factors in hormone receptor-positive, human epidermal growth factor receptor 2 advanced breast cancer in MONARCH 2.10,13
Site of Metastases
- Visceral: lung, liver, pleural, or peritoneal (in the presence or absence of bone metastases).
- Bone only: only in bone.
- Other: other soft tissue sites (in the presence or absence of bone metastases).
Endocrine Resistance (ESO-ESMO guidelines).7,8
- Primary: relapse while on the first 2 years of adjuvant ET, or PD within first 6 months of 1st line ET for MBC, while on ET.
- Secondary: relapse while on adjuvant ET but after the first 2 years, or relapse within 12 months of completing adjuvant ET, or PD ≥ 6 months after initiating ET for MBC, while on ET.
ET: endocrine therapy; HR: hazard ratio; MBC: metastatic breast cancer; PD: progressive disease; 95% CI: 95% confidence interval.
Abemaciclib Improves Progression-Free Survival in Predominantly Chinese Postmenopausal Women with HR+, HER2- Advanced Breast Cancer (MONARCHplus)
Doctor Zefei Jiang
Continuous oral abemaciclib has been approved in combination with ET for patients with HR+, HER2- ABC in more than 50 countries outside of China. Jiang et al.14 conducted the MONARCHplus study to evaluate abemaciclib plus ET in predominantly Chinese patients with HR+, HER2- ABC.14,15 MONARCHplus was a randomised controlled, double-blind, Phase III study for postmenopausal women with endocrine-sensitive (Cohort A) or endocrine-resistant (Cohort B) HR+, HER2- ABC. Cohort A (n=306) received abemaciclib (150 mg q12h) or placebo plus a nonsteroidal aromatase inhibitor (NSAI) (anastrozole or letrozole) as firstline ET. Cohort B (n=157) received abemaciclib (150 mg q12h) or placebo plus fulvestrant following progression to ET. Both cohorts were randomised 2:1 to abemaciclib or placebo.
At the prespecified interim analysis, 119 and 82 PFS events were recorded in Cohorts A and B, respectively. The authors calculated that both abemaciclib plus NSAI and abemaciclib plus fulvestrant were associated with statistically significantly improved PFS and ORR in the ITT population (p<0.0001 for all except p=0.0001 for PFS in Cohort A). In Cohort A, median PFS (primary objective) was not reached with abemaciclib plus NSAI compared with 14.73 months with placebo plus NSAI (HR: 0.499; 95% CI: 0.346–0.719). ORR was 56.0% and 30.3% in the abemaciclib and placebo arms of Cohort A, respectively. In Cohort B, median PFS (secondary objective) was reported to be 11.47 months with abemaciclib plus fulvestrant compared with 5.59 months with placebo plus fulvestrant (HR: 0.376; 95% CI: 0.240–0.588). ORR was 38.5% and 7.5% in the abemaciclib and placebo arms of Cohort B, respectively. The authors found PFS benefit was consistent within all stratification factors and prespecified sensitivity analyses.
The safety profile for both abemaciclib arms was reported to be consistent with previous reports for abemaciclib plus ET, with neutropenia, diarrhoea, leukopenia, and anaemia the most common AE. The authors concluded that abemaciclib in combination with NSAI or fulvestrant provided a statistically significant and clinically meaningful improvement in PFS in predominantly Chinese postmenopausal women with HR+, HER2- ABC.
Abemaciclib Improves Progression-Free Survival in Postmenopausal Women with HR+, HER2+ Advanced Breast Cancer (monarcHER)
Doctor Sara M. Tolaney
Abemaciclib activity in HR+, HER2- ABC is well documented.1,8-11,15 Early clinical data indicate abemaciclib also has activity in HR+, HER2+ ABC, with an ORR of 36% and median PFS of 7.2 months reported in a subset of 11 patients with HR+, HER2+ tumours in a Phase I dose-finding study.16
The authors explained there is also a biological rationale to support abemaciclib activity in HER2+ disease as this inhibitor enhanced the activity of HER2-directed agents and had a synergistic effect in preclinical models.17,18 Furthermore, the addition of HER2-directed therapy to ET modestly improved PFS in patients with HR+, HER2+ ABC.19-21
Given the strong preclinical and clinical data on abemaciclib in anti-HER2 therapy and to further assess the activity of abemaciclib in HR+, HER2+ ABC, Tolaney et al.22 conducted monarcHER, a global, randomised Phase II study of abemaciclib (150 mg taken by mouth q12h on Days 1–21 of a 21-day cycle) plus trastuzumab (intravenously on Day 1 of a 21-day cycle) with (Arm A) or without (Arm B) fulvestrant (500 mg intramuscularly on Cycle 1 Day 1 and Day 15 and Cycle 2 Day 8, then every 28 days) versus trastuzumab plus standard-of-care chemotherapy (Arm C) in postmenopausal women with HR+, HER2+ ABC.22,23 A total of 237 patients were randomised 1:1:1 and stratified by number of prior systemic regimens for ABC and measurable versus nonmeasurable disease. Analysis was performed at 169 events. The authors reported a statistically significant improvement in PFS of 2.6 months for A versus C (primary endpoint: 8.3 versus 5.7 months; HR: 0.673; 95% CI: 0.451–1.003; p=0.0253), and no PFS benefit for B versus C (p=0.385) (Figure 3).22
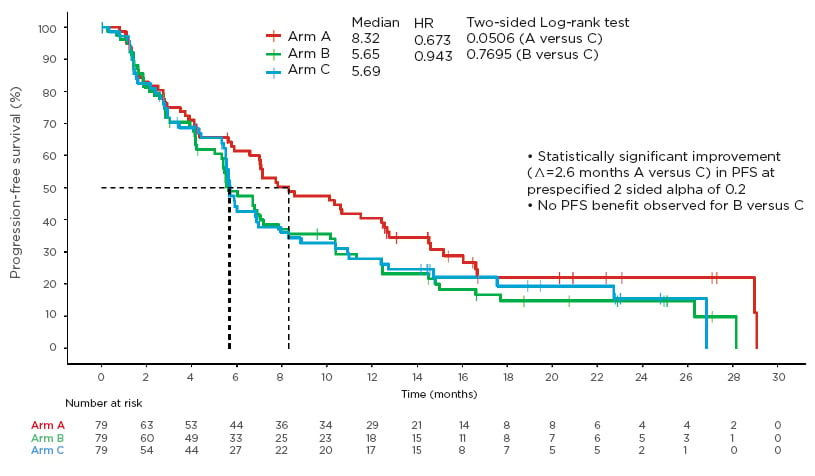
Figure 3: Primary endpoint: progression-free survival.
Progression-free survival in hormone receptor-positive, human epidermal growth factor receptor 2-positive advanced breast cancer in monarcHER.22
HR: hazard ratio; PFS: progression-free survival.
Confirmed ORR for A versus C was 32.9% versus 13.9% for the ITT population and 35.7% versus 15.9% for a subset of patients with measurable disease. There was no difference in ORR between B and C. Importantly, the ORR in Arm A was durable, with a median duration of response of 12.5 months. Safety data were similar to the known safety profile of abemaciclib, with neutropenia, leukopenia, thrombocytopenia, and diarrhoea the most common AE. The authors concluded that in a heavily pretreated population (≥2 prior HER2-directed therapies), the combination of abemaciclib with fulvestrant and trastuzumab led to statistically significant improvement in PFS and ORR compared with standard chemotherapy and trastuzumab, with a 2.6-month absolute improvement in PFS and a more than doubling of confirmed ORR.
Conclusions and Context
The authors’ various approaches to treating HR+ ABC with abemaciclib have yielded clinically meaningful results in the three studies in this review. In MONARCH 2, Sledge et al.10 showed treatment with abemaciclib plus fulvestrant was associated with a statistically significant median OS benefit to premenopausal, perimenopausal, and postmenopausal women with HR+, HER2- ABC who progressed on prior ET. Notably, OS benefits were consistent across all subgroups, including patients with poor prognostic factors such as visceral metastases and primary ET resistance. Bone-only disease data from this study are not yet mature.10 Abemaciclib also significantly delayed the receipt of subsequent chemotherapy in exploratory analysis. Follow-up of MONARCH 2 is ongoing to further characterise OS benefit and exploratory efficacy endpoints. Data from the MONARCHplus interim analysis by Jiang et al.15 indicated that abemaciclib in combination with NSAI or fulvestrant is associated with a statistically significant improvement in PFS in predominantly Chinese postmenopausal women with HR+, HER2- ABC, with the demonstrated benefit consistent with the MONARCH 2 and MONARCH 3 studies.1,8-11 Tolaney et al.22 showed that the combination of abemaciclib with fulvestrant and trastuzumab led to statistically significant improvement in PFS and ORR compared with standard chemotherapy and trastuzumab, and concluded that monarcHER is the first Phase II study to report positive results for a CDK4/6 inhibitor and ET versus standard-of-care chemotherapy, together with HER2-directed treatment, in HR+, HER2+ ABC. Abemaciclib had a generally tolerable safety profile in all three studies, with no new safety signals observed. The safety profile of abemaciclib in these studies was similar to that for other CDK4/6 inhibitors in HR+ ABC.10 Data from these studies, together with previously disclosed data, show abemaciclib has activity in both HR+, HER2- and HR+, HER2+ ABC. Ongoing followup and further clinical studies will continue to add to the clinically important role of abemaciclib in HR+ ABC, including the effects of abemaciclib in TRIPLE+ breast cancer.
To put these results into context, other CDK4/6 inhibitors have also shown significant improvements in PFS versus placebo in key clinical trials in patients with HR+, HER2- ABC. In PALOMA2, in postmenopausal patients with HR+, HER2- ABC who had not had prior treatment for advanced disease, median PFS was 24.8 versus 14.5 months for palbociclib versus placebo (both with letrozole) (HR: 0.58; 95% CI: 0.46–0.72; p<0.001).24 The results from PALOMA3, in which palbociclib or placebo was used in combination with second-line fulvestrant in patients with HR+, HER2- ABC who had progressed on ET, showed PFS was 9.5 versus 4.6 months for palbociclib versus placebo (HR: 0.46; 95% CI: 0.36–0.59; p<0.0001).5,25 Ribociclib has been shown in the MONALEESA studies to significantly improve PFS compared with placebo. These studies include MONALEESA 2 which showed 25.3 versus 16.0 months (HR: 0.568; 95% CI: 0.457–0.704) with letrozole in postmenopausal women with HR+, HER2- ABC;26-28 20.5 versus 12.8 months (HR: 0.593; 95% CI: 0.480–0.732; p<0.001) in MONALEESA 3 with second-line fulvestrant in patients with HR+, HER2- ABC reported in 2018, and 33.6 versus 19.2 months (HR: 0.546; 95% CI: 0.415–0.718) reported in 2019;29,30 and 23.8 versus 13.0 months (HR: 0.55; 95% CI: 0.44–0.69; p<0·0001) in MONALEESA 7 with tamoxifen/NSAI and goserelin in premenopausal patients with HR+, HER2- ABC.31 Although the individual data from these studies cannot be directly compared because of population differences, and there have been no head-to-head studies, the data are in line with those from MONARCH 2 and MONARCHplus in this review and confirm the PFS benefits of CDK4/6 inhibitors in HR+, HER2- ABC.
OS data from MONALEESA 7 showed that ribociclib plus ET was associated with clinically and statistically significantly longer OS than ET alone in premenopausal patients with HR+, HER2- ABC (median OS: not reached versus 40.9 months in the ribociclib versus placebo arms, respectively; HR: 0.712; 95% CI: 0.54–0.95; p=0.00973).32 These results are supported by those from MONALEESA 3, in which median OS was not reached versus 40.0 months for the ribociclib and placebo arms, respectively (HR: 0.724; 95% CI: 0.568–0.924; p=0.00455).30 In PALOMA 3, there was clinically meaningful but not statistically significant improvement in OS with palbociclib compared with placebo in combination with fulvestrant in second and further-line-treated patients: median OS was 34.9 months in the palbociclib-fulvestrant group and 28.0 months in the placebo-fulvestrant group (HR: 0.81; 95% CI: 0.64–1.03; p=0.09; absolute difference: 6.9 months).33
The OS findings from MONALEESA 3, MONALEESA 7, and PALOMA 3 are consistent with those from MONARCH 2 in this review and show that CDK4/6 inhibitors are associated with clear and meaningful clinical benefit for patients with HR+, HER2- ABC. The clear survival benefit with abemaciclib in HR+, HER2- ABC patients with visceral metastases in MONARCH 2 was also seen with ribociclib in this patient subgroup in CompLEEment-1, an openlabel, Phase IIIb study evaluating ribociclib and letrozole as firstline therapy in an expanded population (clinical benefit rate: 62.8%; 95% CI: 60.3–65.2).34 Overall, these data show that CDK4/6 inhibitors improve PFS in the first and secondline setting in HR+, HER2- ABC and this translates into an improvement in OS, with clear survival benefits observed in patient subgroups that have a poorer prognosis.

![EMJ Oncology 8 [Supplement 2] 2020](https://www.emjreviews.com/wp-content/uploads/2020/08/EMJ-Oncology-8-Supplement-2-2020-Feature-Image-940x563.jpg)






