Meeting Summary
Returning to in-person for the first time since the COVID-19 pandemic started, the European Society for Medical Oncology (ESMO) 2022 conference was packed with new and interesting data. Highlights include updates from DESTINY-Gastric02 and DESTINY-Lung02 exploring treatment with trastuzumab deruxtecan in different settings, and new and encouraging results for the KRAS inhibitors adagrasib and the first-in-class sotorasib in advanced colorectal cancer (CRC) and in previously treated patients with non-small cell lung cancer (NSCLC). Of note, updates from APPLE and INSIGHT2 trials strengthen the application of liquid biopsy for disease monitoring and recurrence, while the PATHFINDER study results opened a fierce debate in the medical community, with many stakeholders suggesting that liquid biopsy is not yet fully ready for prime time in early cancer detection. Nonetheless, the one thing that everybody agreed on, and was vividly supported by almost all presenters this year, is the unmet clinical need related to molecular profiling of patients with cancer. While targeted next-generation sequencing (NGS), mostly in the form of gene-specific panels rather than whole exome sequencing, has emerged as the absolute game-changer for precision medicine concrete realisation, the medical community urgers investments aimed to build NGS testing capability ultimately enabling oncologists to pick the best treatment path for their patients. At ESMO Congress 2022 numerous studies highlighted the remarkable steps that precision medicine has made during the last year.HER2: A Phenomenal Success Story that Endures in 2022
Accumulating success continues for trastuzumab deruxtecan, targeting HER2, following positive readouts across various indications presented at ESMO, including gastric and NSCLC. In the Phase III DESTINY-Gastric02 trial, investigators reported an impressive overall response rate (ORR) of 41.8%, with 5% of these being complete responses, solidifying trastuzumab deruxtecan journey towards becoming the new standard of care in second-line trastuzumab-pre-treated HER2-positive recurrent or metastatic gastric cancer.1
For NSCLC, top-line results from the Phase II DESTINY-Lung02 trial support trastuzumab deruxtecan’s recent approval in the USA for previously treated patients with unresectable or metastatic NSCLC with HER2 mutations, approximately 2–4% of all patients with NSCLC, who often present with a poor prognosis. Patients in DESTINY-Lung02 had previously received platinum-based chemotherapy, and data showed a 53.8% objective response rate.2 These results bode well for the confirmatory Phase III DESTINY-Lung04 trial testing trastuzumab deruxtecan as a first-line treatment for patients with unresectable or metastatic NSCLC harbouring HER2 exon 19 or 20 mutations and should support its rapid uptake in HER2-mutated NSCLC (unpublished data, presented at ASCO Annual Meeting, 3-7 June, 2022).
On the breast cancer front, with more than two-thirds of cases being hormone receptor-positive (HR+)/HER2-negative, ESMO brought good news about two different targeted therapies for this subtype. The effect of cyclin-dependent kinase 4 and 6 inhibition with the new drug dalpiciclib was tested in a Phase III trial, DAWNA-2, in the first-line setting.3 Dalpiciclib nearly doubled progression-free survival (PFS) of patients with untreated HR+/HER2-negative breast cancer, regardless of menopausal status, when given with letrozole or anastrozole as a first-line treatment. Moreover, final results from the monarcHER trial demonstrated improved overall survival (OS) with abemaciclib, in combination with HER2-targeted therapy (trastuzumab) with or without hormonal therapy compared with chemotherapy plus trastuzumab in patients with HR+, HER2-positive advanced breast cancer.4 These final data suggest that a triple-agent, chemotherapy-free treatment regimen may improve OS in patients with HR+, HER2-positive advanced breast cancer, a disease setting in which treatment options are limited.
KRAS: Targeting the “Undruggable,” Small Steps but Still a Lot to Do
Focus on Colorectal Cancer
Expectations were nothing but huge at this year’s ESMO meeting for KRAS inhibitors. Updated results from the Phase I CodeBreaK 101 trial that assessed double combination of KRAS and EGFR blockade with sotorasib and panitumumab for advanced CRC KRASG12C positive patients, showed an ORR of 30% of patients and a median response of 4.4 months.5 In line with these results, updated data from the Phase I/II KRYSTAL-1 study confirmed that adagrasib in combination with cetuximab (double blockade of KRAS and EGFR) in heavily pre-treated patients results in an ORR of 46% and a median PFS of 6.9 months.6 Bearing in mind that until recently, KRAS was considered an undruggable target, these data reinforce the potential for mutant-selective KRAS inhibitors in combination with anti-EGFR agents in advanced CRC.
Focus on Lung Cancer
While 3% of patients with CRC have KRASG12C mutation, around 12% of patients with NSCLC harbour this mutation. Sotorasib was the first therapy to receive approval for the treatment of patients with KRASG12C-mutated NSCLC in May 2021, a notoriously hard-to-treat patient population with high unmet needs. At ESMO, a confirmatory Phase III CodeBreaK 200 trial was presented. Sotorasib met its primary endpoint delivering a significant benefit in median PFS over chemotherapy; however, it failed to show a statistical and numerical benefit in OS.7 While the Phase III readout should support the uptake of this therapy as the new standard of care and may be enough to secure conversion of its conditional approval to full approval, it is clear that overall, the numerical data for PFS and OS released from CodeBreaK 200 are below expectations. Furthermore, concerns about drug toxicity will have to be addressed carefully for sotorasib, thus its path forward is still uncertain. The underwhelming numerical results and lack of survival benefit suggest that different kinds of therapeutic combinations may be explored in the near future to significantly impact patients’ clinical outcome.
Liquid Biopsy Takes the Scene in Therapy Resistance and Disease Monitoring
Disease Monitoring via Circulating Tumour DNA Makes Sense
Resistance to tyrosine kinase inhibitors (TKIs) of the EGFR treatment in advanced NSCLC represents a major therapeutic challenge. The underlying mechanism can be EGFR-dependent, including the T790M mutation, or driven by independent molecular pathways, like HER2 and MET amplifications.
At ESMO Congress 2022, key updates on the use of liquid biopsies for detecting mutant circulating tumour DNA (ctDNA) that may have a major potential in the clinical space were discussed. For example, the results of the APPLE trial supported that serial monitoring of T790M status by ctDNA is feasible in patients with EGFR-mutated advanced NSCLC and should be used to drive treatment decisions.8 This strategy identified 17% of patients with molecular progression before response evaluation criteria in solid tumours progressive disease, leading to an earlier switch in favour of osimertinib. Moreover, ctDNA clearance is an early predictor of favourable outcomes on treatment with gefitinib. Altogether, these results indicate that serial ctDNA assessment comes with a clear clinical value, and may be best utilised for risk-adaptive treatments.
Investigating Resistance Using Liquid Biopsy Has a Clear Potential
Between 15–30% of patients with EGFR-mutated NSCLC and treated with osimertinib develop resistance through MET amplification, and this is associated with poor prognosis.
The two-arm Phase II INSIGHT 2 study addressed the response to the combination of tepotinib plus osimertinib for patients with advanced EGFR-mutated NSCLC with MET amplification that recurred after first-line osimertinib.9 About 425 subjects with advanced EGFR-mutated NSCLC were screened for MET amplification following progression on the TKI osimertinib. Of these, 25% resulted positive for MET amplification via fluorescent in situ hybridisation in tissue biopsy samples, 3% were positive by NGS liquid biopsy, and 8% had MET amplification with both methods. In the group treated with tepotinib plus osimertinib and with MET amplification detected by liquid biopsy, about 50% of participants with at least 9 months of follow-up had a complete or partial response, while the ORR of patients treated with tepotinib monotherapy was only 8.3%. These findings strongly indicate that addressing resistance mechanisms can improve patient outcomes and that NGS-based liquid biopsy testing may untangle unknown resistance mechanisms.
Minimal Residual Disease to Predict Recurrence Benefits from Circulating Tumour DNA Testing
Many are wondering when liquid biopsy will eventually be accepted as a tool to more accurately predict recurrence, especially in CRC. Previous evidence indicated that restricting adjuvant chemotherapy to ctDNA-positive and not ctDNA-negative patients with resected Stage II CRC reduced unnecessary chemotherapy exposure without compromising recurrence-free survival.10 The new data of the DYNAMIC trial presented at ESMO Congress 2022 showed that post-operative ctDNA analysis was more sensitive than standard methods (intensive imaging via CT scanning) for predicting distant recurrences.11 Moreover, ctDNA clearance was achieved with adjuvant chemotherapy in 87% of post-operative patients who were ctDNA positive and this predicted favourable outcome (2-year recurrence free survival: 97%) compared with patients without ctDNA clearance (1-year recurrence free survival: 20%). Interestingly, neither post-operative nor post-chemotherapy carcinoembryonic antigen (CEA) levels add prognostic value for patients who were ctDNA negative. In line with these data, the Phase III PRODIGE 13 trial showed a lack of survival advantage with intensive follow-up (CT scan and CEA) in patients with resected Stage II–III CRC when looking at the whole study population.12 Therefore, ctDNA analysis allows to distinguish between patients at greater risk of recurrence who may benefit from adjuvant treatment and those at lower risk who could receive surgery alone. The classical marker for CRC, CEA, lacks sensitivity and specificity for recurrence and it will probably be replaced by ctDNA in future. Although for a limited number of specific clinical scenarios, minimal residual disease is making its way into routine clinical practice.
Next Generation Sequencing Is Uniquely Bonded to Precision Medicine, No More Proof Is Needed
On top of key scientific sessions highlighting the value of molecular profiling, a number of industry symposia equally underlined the effectiveness of NGS biomarker testing in driving therapy selection. Despite being an imperative aspect of target therapy access, there are still several challenges to a much broader implementation of NGS into clinical practice, including limited technology access, inequalities in healthcare reimbursement as well a lack of technical expertise, and duly trained personnel. Remarkable examples paving the way to overcome these inequalities have been shown at ESMO Congress 2022, including an update on the European Program for ROutine testing of Patients with Advanced lung cancer (EPROPA), which was presented at the World Conference on Lung Cancer (6th–9th August 2022). This initiative aimed to deliver diagnostic and therapeutic opportunities for underserved patients across Europe, and has demonstrated the feasibility to provide a comprehensive and clinically meaningful NGS-based molecular characterisation for patients with cancer, by means of optimising biopsy material management combined with a markedly reduced diagnostic turnaround time. Indeed, access to biomarker testing and availability of test results prior to first-line therapy has been shown to directly impact patient survival, thus supporting the implementation of ultra-fast in-house NGS testing for better clinical outcomes.13
A pivotal example of how to establish a highly automated end-to-end workflow for ultra-fast NGS biomarker testing is ongoing at the Molecular Diagnostic Laboratory of the Hospital del Mar in Barcelona, Spain (Figure 1), and was showcased at ESMO Congress 2022. The overarching aim at the Hospital del Mar is to implement an integrated NGS workflow that provides an assessment of clinically actionable mutations from both tissue and liquid biopsy samples while delivering lab results within just 1 day. Though this might appear to be an ambitious goal, an CE in vitro diagnostic compliant NGS technological solution exists nowadays on the market, and can deliver on the promise of ultra-fast simultaneous multiple biomarker testing of actionable genes for therapy selection, ensuring that no patient is left behind.
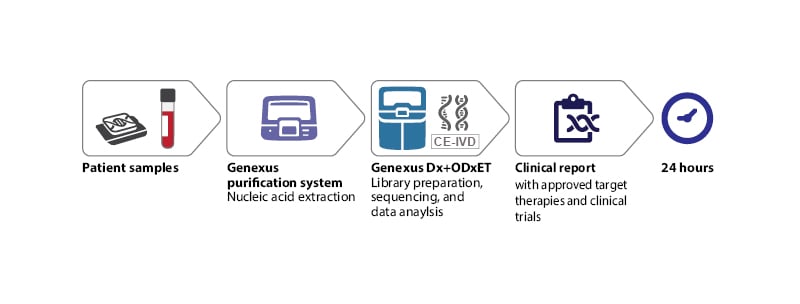
Figure 1: Optimal next-generation sequencing workflow for routine diagnostics in oncology.
CE-IVD: CE in vitro diagnostic; ODxET: Oncomine Dx Express Test.
Liquid Biopsy for Early Cancer Detection: Moving Forward but Not Ready for Prime Time
Despite the ESMO Congress 2022 highlighting the potential of liquid biopsy for disease monitoring and recurrence, its application for early cancer detection or screening is still far from its full clinical implementation.
In the prospective PATHFINDER study, a multi-cancer early detection blood assay used a targeted methylation NGS based test to detect and analyse ctDNA aiming to predict cancer presence ‘molecular signals,’14 i.e., the presence of genomic alterations associated with tumour occurrence. The study included 6,621 adults aged 50 and older who were divided into two cohorts. One group was deemed high-risk if they had a history of cancer but had been disease-free for at least three years, had smoked more than 100 cigarettes in their lifetime, or had a genetic risk of cancer. A cancer signal was detected in 92 individuals. Follow-up ended at 12 months for both groups. Among those who tested positive, cancer was found in 35 people while the other 57 had no detectable cancer. Cancer was confirmed in 35 of these, giving a positive predictive value of 38.0%. This is a drop from the 44.6% figure from the interim cut reported at last year’s American Society of Clinical Oncology (ASCO). Conversely, a high (98.6%) negative predictive value was observed; the cancer signal origin prediction accuracy was also high at 88.0%. Notably, for false positive results, the time to diagnostic resolution for participants was on average 162 days. This extremely long waiting time may cause anxiety and distress in individuals where the multi-cancer early detection signal was detected. The number and types of subsequent tests required for diagnostic resolution, along with the associated costs, are equally concerning. Altogether these results indicate that it is premature to imagine a clear benefit for this technology applied to population-wide screening. Another prominent study presented at ESMO was the PROMISE trial. This is a prospective multicentre case-control study to assess the performance of a multi-omics approach including ctDNA methylation, mutations, and protein biomarkers in the early detection of nine cancer, including CRC, lung, and ovary tumours.15 Blood samples were prospectively collected from 511 cancer patients and 470 non-cancer controls. Multi-omics model resulted in 98.3% specificity and overall sensitivity of 83.7%, with 71.4% for Stage I cancers and 93.6% for advanced Stage IV tumours. Therefore, sensitivity and specificity of a multi-cancer detection blood test were high for the detection of cancers, and suggest that for future clinical application multi-omics approach may result in higher specificity for early cancer detection.
Takeaway from ESMO Congress 2022: New Drugs Uptake Needs More Patients Testing
It is ever clear from this year’s ESMO Congress that the promise of precision medicine will not turn into reality unless a widespread use of molecular profiling for patients with cancer will be eventually implemented at large scale. The pipelines for new therapies associated with specific biomarkers continue to grow steadily, with about 100 new oncology drugs or drug combinations to be launched within the next 3–5 years. Still, as of today, access to testing and thus to new drugs is only a privileged option for a few national healthcare systems, while most are lacking behind. Health policymakers, medical institutions, manufacturers, clinicians, and biomedical researchers, along with patients’ associations, have engaged and joined forces, aiming to push for a much broader NGS adoption through global initiatives. Nonetheless, salt and pepper adoption of testing is still the norm.16 A concrete example lies for instance with poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitors is able to achieve impressive clinical results but still missing an accessible and flexible solution for homologous recombination deficiency testing, leading to low adoption rates, and patient leakage. The need to include biomarkers testing readiness in the process of a new drug launch and having sufficient runway to enable laboratories’ appropriate preparation is paramount to realise the full value of precision medicine.
Groundbreaking technological solutions are now available to tackle these issues, enabling fast and accurate multi-biomarker testing using NGS panels like never before. Investments in new diagnostic capabilities, from infrastructure to equipment and duly trained personnel, need to be viewed as an integral part to reshape and uplift the cancer care continuum workflow.








