Meeting Summary
The symposium took place during the 2024 European Society for Medical Oncology (ESMO) Congress in Barcelona, Spain, with the goal of highlighting the synergy between tissue and liquid biopsy testing in the diagnosis and treatment of solid tumours.
Christian Rolfo, Director of the Division of Medical Oncology at The Ohio State University Comprehensive Caner Centre, Columbus, USA, set the stage with a discussion on the state-of-the-art liquid biopsy for solid tumour testing. He was followed by Guilhem Roubaud from the Bergonié Institute in Bordeaux, France, and Federico Cappuzzo from Regina Elena Institute, Rome, Italy, who delved into molecular testing challenges and applications in genitourinary and non-small cell lung cancer (NSCLC), respectively. Practical insights on next-generation sequencing (NGS) implementation was provided by Bence Sipos, TBAG of Molecular Pathology Baden-Württemberg, Germany, with real-world case studies presented by Sara Pilotto, University Hospital, Verona, Italy, and Roubaud.
The faculty emphasised the importance of integrating liquid and tissue biopsies to perform tumour molecular profiling, which is crucial for accurate diagnosis and personalised treatment strategies. They also highlighted the critical role of rapid NGS in detecting a wide range of genetic alterations to enhance the precision of diagnosis and access to precision therapies.
Introduction
While tissue biopsy remains the gold standard method for obtaining pathological information, it has limitations such as difficulty in acquiring adequate and sufficient tissue and capturing tumour heterogeneity.1 Liquid biopsy, particularly circulating tumour DNA (ctDNA), provides advantages such as the ability to sample over time, high concordance with tissue analysis, and insights into tumour evolution.1
As precision medicine continues to evolve, the integration of cutting-edge techniques such as liquid biopsy and NGS is revolutionising the way cancer is being diagnosed and treated.1 This symposium provided a platform for renowned oncologists, pathologists, and researchers to share their insights and discuss the applications of these technologies in clinical practice.
Layering Diagnostic Steps May be Able to Shorten the Diagnostic Odyssey
Christian Rolfo
Multiple organisations, including the International Association for the Study of Lung Cancer (IASLC) and ESMO, have recommended the use of tumour NGS for the molecular analyses of patients with advanced cancers, including NSCLC, in routine practice.2 Rolfo outlined three strategies for the use of liquid biopsy in treatment-naïve advanced NSCLC setting.1
- Sequential approach: utilising liquid biopsy when tissue samples are inadequate or unavailable.
- Complementary approach: employing both tissue and liquid biopsy simultaneously.
- Plasma-first approach: prioritising liquid biopsy, progressing to tissue if negative.
Rolfo emphasised that the complementary approach is often the most beneficial, providing a holistic view of the patient’s molecular profile from the outset. Additionally, incorporating liquid biopsy early in the diagnostic process can shorten the time required to achieve a definitive diagnosis and therefore to initiate appropriate therapy.3 Rolfo presented data (Figure 1) showing that liquid biopsy, when used at baseline or during clinical suspicion, can provide quick identification of actionable genetic alterations, thereby allowing for faster initiation of targeted therapies, ultimately reducing the diagnostic odyssey often faced by patients with cancer.3
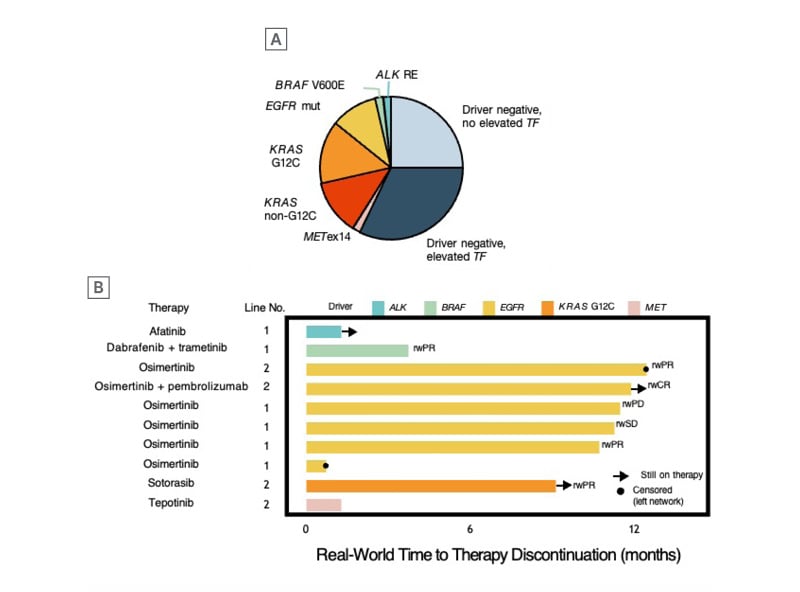
Figure 1: Genomic results and treatment outcomes in pre-diagnosed liquid biopsy cohort.
A) Of 56 patients with liquid biopsy testing ordered before confirmed diagnosis, 24 patients (43%) harboured oncogene drivers. Among driver-negative patients, an elevated cfDNA tumour fraction ≥1% was observed in 18 patients (true-negative cases), while the others (n=14) had no elevated ctDNA tumour fraction (<1%) and were potentially false-negative cases. B) Among the 56 cases, 42 had documented the line of therapy received, and 10 driver-positive patients received matched therapy with real-world response in 71% of the cases.
cfDNA: cell-free DNA; ctDNA: circulating tumour DNA.
Adapted from Russo et al.3
Earlier Testing is Better, Particularly for BRCA-Altered Patients
Guihem Roubaud
Roubaud provided an in-depth analysis of molecular testing in genitourinary oncology. Molecular testing in prostate cancer and bladder cancer is crucial for identifying genetic alterations that inform prognosis, guide treatment decisions, and provide opportunities for personalised therapy.4,5 The timing and method of testing are essential to maximise the benefits of these advanced diagnostic tools.4
Prostate Cancer
In advanced prostate cancer, Roubaud highlighted the critical role of identifying homologous recombination repair (HRR) gene alterations, such as BRCA1/2 mutations.4 These genetic alterations are associated with poor prognosis and can predict responsiveness to poly ADP ribose polymerase (PARP) inhibitors, which have been shown to improve radiographic progression-free survival and overall survival in patients with BRCA1/2 alterations.6-12 However, important factors must be considered.13
- Recent biopsies preferred: recent tissue biopsies are preferable to older samples for molecular testing as these are more likely to provide accurate and relevant genetic information.13
- Extra-bone versus bone biopsies: biopsies from extra-bone localisations are generally more effective than bone biopsies. This is significant because a substantial number of patients may present with bone-only metastases, thus, special care must be taken during the decalcification process of bone biopsies to avoid DNA denaturation.13
- Germline versus somatic mutations: if a somatic mutation is not detected in the tissue biopsy, germline testing can be performed. However, Roubaud highlighted that relying solely on germline testing may miss somatic-only mutations, which can be crucial for treatment decisions.13
- Liquid biopsy testing: cell free deoxyribonucleic acid (ctDNA) analysis has shown good concordance with tissue and should be performed in the case of disease progression. Liquid biopsy testing should be performed before the initiation of androgen deprivation therapy, as ctDNA concentration rapidly decreases during effective treatment.13
Bladder Cancer
In bladder cancer, the precise molecular characterisation through tissue biopsies is becoming increasingly important to optimise therapy selection. This is essential for identifying genetic alterations such as FGFR3 mutations and fusions, which can guide the use of targeted therapies like erdafitinib (an FGFR tyrosine kinase inhibitor for treatment of unresectable or metastatic urothelial cancer in those with an FGFR3 alteration who have worsened on prior immunotherapy)14 in the metastatic urothelial carcinoma (mUC) setting.15
There are various techniques for molecular testing of FGFR3 alterations, including polymerase chain reaction (PCR) and NGS. However, obtaining high-quality tissue samples, particularly in the advanced stages of the disease remains a challenge.16
While emphasising the importance of tissue biopsies, Roubaud also highlighted the role of liquid biopsy as a complementary tool. Liquid biopsy can be particularly useful when tissue samples are not available or are insufficient for molecular analysis. As Roubaud summarised, liquid biopsy can help monitor tumour dynamics and detect resistance mechanisms, although it may present challenges for the accurate detection of fusions and should ideally be confirmed with tissue biopsy results when possible.
The Importance of a Complete Molecular Profile for All Patients with Non-Small Cell Lung Cancer, Regardless of the Stage
Federico Cappuzzo
Cappuzzo provided a thorough examination of the evolving landscape of NSCLC treatment, emphasising the critical role of molecular testing and the integration of liquid biopsy in clinical practice.
Neoadjuvant Chemoimmunotherapy
The transition from surgery alone to neoadjuvant chemoimmunotherapy for resectable NSCLC shows promise as a treatment strategy; however, the benefit of this treatment approach is not universal, particularly for patients with oncogene-addicted tumours such as EGFR mutations, who do not benefit from the addition of immunotherapy.17,18
Adjuvant Immunotherapy
For patients who have already undergone surgery, Cappuzzo highlighted the role of adjuvant immunotherapy. He stressed the importance of identifying patients who are most likely to benefit from adjuvant immunotherapy, particularly those with high levels of PD-L1 expression. Conversely, oncogene-addicted patients, such as those with EGFR mutations, benefit more from targeted therapies in the adjuvant setting.19,20
Unresectable Non-Small Cell Lung Cancer
For unresectable NSCLC, the current standard of care is concomitant chemoradiation followed by durvalumab, based on the PACIFIC trial results.21 Cappuzzo noted that the benefit of adding durvalumab is not seen in patients with oncogene-addicted tumours, highlighting the need for molecular profiling to guide treatment decisions.
The Role of Liquid Biopsy
Liquid biopsies present several applications such as screening or identifying patients who have achieved a complete pathological response and may not require further treatment, thereby reducing the risk of overtreatment and associated toxicities. Additionally, there is potential in liquid biopsy to identify minimal residual disease and monitor treatment response, given that patients with detectable ctDNA after surgery are at a higher risk of relapse and may benefit from intensified adjuvant therapy (Figure 2).22
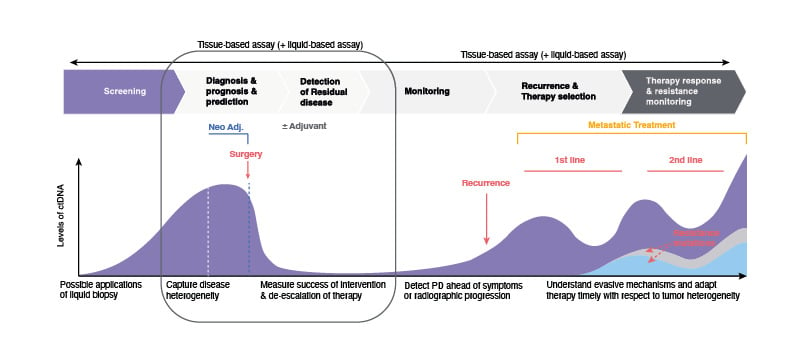
Figure 2: Applications of circulating tumour DNA analysis.
ctDNA: circulating tumour DNA; NSCLC: non-small cell lung cancer; PD: progressive disease.
Adapted from Wan et al.22
Liquid biopsy, when used alongside tissue biopsy, can provide a more comprehensive molecular profile of the tumour. Cappuzzo’s case studies illustrated the successful application of molecular testing and liquid biopsy for timely and accurate molecular diagnostics in personalising treatment and achieving better patient outcomes.
A Quiet Revolution has Been Unfolding in the Field of Next-Generation Sequencing
Bence Sipos
Sipos provided a practical overview of the implementation of NGS in clinical practice. From a pathologist’s perspective, the key clinical needs include:23
Molecular Characterisation
Pathologists are tasked with providing molecular profiling of malignant tumours, which is essential for identifying actionable genetic alterations. However, factors to consider include the molecular biomarkers specific to each tumour type and the reimbursement for testing in specific countries or regions.
Timely and Accurate Diagnosis
Rapid turnaround time (TAT) is crucial for timely clinical decision-making. Studies have demonstrated that upfront characterisation of lung cancer is associated with survival benefits.24,25 Pathologists must ensure that NGS results are delivered within a few days to meet the needs of multidisciplinary tumour boards (MTB) and enable prompt treatment initiation.
High Reliability and Low Failure Rates
Sipos emphasised that ensuring the reliability of NGS results is paramount. He noted that pathologists strive to achieve low failure rates in molecular testing to provide clinicians with accurate and actionable data. In his presentation, Sipos highlighted that his laboratory achieved a failure rate of less than 1%, demonstrating the importance of robust and reliable testing protocols.
Pathologists also must efficiently utilise available tissue samples, especially when dealing with small biopsies or suboptimally processed samples.
Navigating Molecular Diagnostics
Sipos highlighted the diverse choices available to pathologists in molecular diagnostics.23 PCR is suitable for detecting specific, predefined genetic alterations and is quick and cost-effective; however, it is limited to a small number of targets, so it may be more suited for follow-up testing of known variants.
Within NGS, the target enrichment method has implications for clinical testing.26 Hybrid-capture NGS is highly flexible and capable of detecting an unlimited number of targets; however, it requires a relatively high amount of input DNA of 40–80 ng and involves a longer TAT due to a more complex workflow.
Amplicon-based NGS, which requires less input DNA, is particularly valuable for routine clinical diagnostics. While there is limited flexibility in target selection, less input DNA (as low as 5–20 ng) makes it suitable for small biopsies and degraded samples, and faster TAT is possible, with some methods delivering results in as little as 2 days.
By selecting the appropriate methods, optimising sample handling, ensuring rapid and reliable results, and collaborating effectively with clinical teams, pathologists can significantly contribute to the success of personalised cancer treatment. Using an amplicon-based NGS panel, Sipos stated that with his team they can analyse both tissue and liquid biopsy samples, achieving a TAT of up to 3 days in 82% of cases and a failure rate of <1% (Figure 3). In his lab, the reliability, failure rates, and TATs of amplicon-based NGS are equivalent to routine methods in pathology, such as immunohistochemistry, resulting in optimal decision-making and patient care.
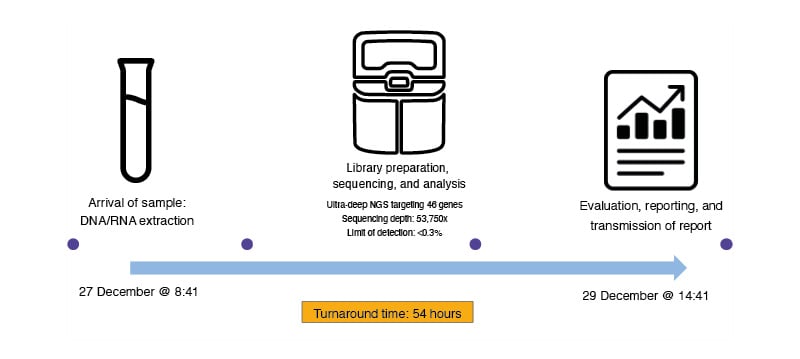
Figure 3: Timeline of liquid biopsy testing from Sipos’ lab.
The process includes the arrival of the sample, DNA/RNA extraction, ultra-deep NGS targeting 46 genes with a sequencing depth of 53,750x and an LOD of <0.3%, followed by data evaluation, report generation, and transmission of the final report in 54 hours.
LOD: limit of detection; NGS: next generation sequencing.
Adapted from Sipos’ presentation at the symposium.
Case Studies Illustrate the Importance of Adequate Molecular Profiling
Three case studies were presented by Roubaud and Pilotto. These highlighted the importance of comprehensive molecular profiling using both liquid and tissue biopsies at diagnosis to avoid delays in identifying actionable mutations and making appropriate treatment decisions in prostate, bladder, and lung cancer.
Conclusion
The symposium concluded with a panel discussion featuring all the presenters, where Pilotto emphasised the complementarity of liquid and tissue biopsy and their high concordance and ability to reduce time to treatment in her clinical practice. The symposium concluded with closing remarks from Rolfo, who emphasised the importance of continued collaboration and innovation in the field to improve patient outcomes.
EM-167676
October 2024







