Meeting Summary
The metastatic non-small cell lung cancer (mNSCLC) treatment landscape has vastly expanded over the past two decades as a result of advancements in biomarker testing. However, unmet needs remain both in terms of treatment options for some patient groups, and patient support throughout the treatment journey. In this symposium, Jarushka Naidoo, Consultant Medical Oncologist, Beaumont RCSI Cancer Centre, Dublin, Ireland; Terri Conneran, KRAS Kickers, Charlotte, North Carolina, USA; Luis Paz-Ares, Chair of the Medical Oncology Department, Hospital Universitario 12 de Octubre, Madrid, Spain; and Alexander Drilon, Chief of Early Drug Development and Thoracic Oncology, Memorial Sloan Kettering Cancer Center, New York, USA, focused on patient-centric approaches to mNSCLC treatment, starting with a patient and patient advocacy group perspective on what patients want from their care team during their treatment journey. The panel also discussed both immuno-oncology (I-O) monotherapy and combination therapy, including dual I-O therapies for patients with programmed death-ligand 1 (PD-L1) tumour expression <1%, as well as the treatment landscape for KRASG12C-mutated mNSCLC, and ongoing trials of KRAS-targeted agents. In addition, the latest data on tyrosine kinase inhibitors (TKI) for patients with alterations in ROS1 and NTRK genes were discussed, focusing on next-generation TKIs. Finally, the panel discussed patient cases, taking into account specific considerations and how to best approach treatment decisions.
Introduction
Naidoo provided an introductory overview of the mNSCLC treatment landscape and its vast expansion over the past two decades.1-4 In addition, she highlighted the multitude of targetable biomarkers in mNSCLC while underscoring the importance of treating patients with the appropriate therapy. However, while testing can help change patient outcomes, the important role of other members of the multidisciplinary team and patient advocacy groups should not be overlooked.5
The Importance of Patient Empowerment During Their Journey
Conneran introduced herself as a lung cancer survivor of 7.5 years and gave an overview of her personal cancer journey, which included five separate recurrences. She highlighted that it was only after she experienced two recurrences and received additional opinions that she was diagnosed with a KRAS-mutated tumour. Upon researching KRAS mutations, she recalled feeling scared after finding out that her KRASG12C cancer was described as ‘undruggable’. This motivated her to seek knowledge, and connect with other individuals with KRAS-mutated cancers.
KRAS Kickers
Conneran explained that patients tend to connect to information that is available to them and that without it, they feel very vulnerable, especially in terms of deciding next steps. As a result, Conneran founded KRAS Kickers in January 2020, initially as a Facebook group, to connect with other patients experiencing the same issues worldwide. In particular, she used this opportunity to learn more about KRAS mutations, what they mean for patients, and why patients should care. Patients within the group were seeking similar information regarding their cancer, including Knowledge, Research, Advocacy, and Survivorship (KRAS). In doing so, KRAS Kickers sought to use the term ‘KRAS’ as a call to action for patients and not just a driver of their cancer. They also introduced the term ‘KRAS HOLEs’; gaps in, or barriers to, optimal Healthcare Outcomes Literacy and Equity (HOLE), which includes full biomarker analyses and result sharing with patients.
Patient Empowerment
Patients need to be involved in treatment decisions and feel empowered. Patient empowerment involves sharing new information from a rapidly evolving field with patients directly to help identify what they need to know and enable them to take the next steps. It is crucial for patients to be able to make decisions strategically so that they can make the right decisions for themselves in the future, such as choosing to take part in a clinical trial or taking their own personal next steps. Physicians need to share information with patients, such as what their cancer is and what is driving it, to help eliminate the anxiety that patients feel. Conneran reminded the audience that patients trust physicians with their lives at a time when they are potentially the most scared and vulnerable.5
Conneran concluded the session with a summary of how KRAS Kickers is empowering over 10,000 patients in 117 countries, and highlighted the importance of connecting with patient groups, providing examples of other biomarker-specific patient groups (Figure 1).
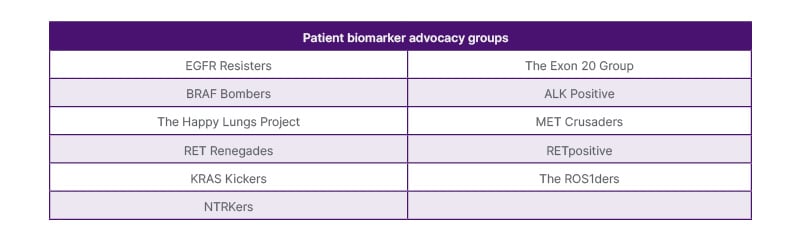
Figure 1. Biomarker patient advocacy groups.
PD-L1 <1% in Metastatic NSCLC: Guiding Treatment Decisions for Potential Durable Outcomes in a Patient Population with Unmet Needs
Paz-Ares, Hospital Universitario 12 de Octubre, Madrid, Spain, set the scene with an overview of the increasing number of first-line (1L) treatment options available for patients with mNSCLC and no targetable mutations, including I-O monotherapy and combination therapy with chemotherapy, describing I-O as the cornerstone of treatment for these patients. He highlighted that, while I-O monotherapy is an option for patients with high tumour PD-L1 expression, a combination of I-O therapy and chemotherapy may be the most suitable option for other patients, including those tumors without PD-L1 expression.3,4
Outcomes for Patients with Tumour PD-L1 <1%
There remains an unmet need for treatments with long-lasting efficacy in patients with tumour PD-L1 expression <1%.6 In the KEYNOTE-189 trial, an overall survival (OS) benefit with pembrolizumab + chemotherapy was observed across all levels of tumour PD-L1 expression versus placebo + chemotherapy. At year 5, OS rates were 19% and 10%, for pembrolizumab + chemotherapy, in the ITT population and those with tumour PD-L1 expression <1%, respectively. However, within 3 years, most patients with tumour PD-L1 expression <1% had experienced disease progression, with a median progression-free survival (PFS) of 6.2 months for pembrolizumab + chemotherapy versus 5.1 months for placebo + chemotherapy (hazard ratio [HR]: 0.67; 95% CI: 0.49–0.92).7 This was followed by 5-year data from a pooled analysis of results from KEYNOTE-189 and KEYNOTE-407, in which limited clinical benefit was shown for 1L pembrolizumab + chemotherapy treatment for patients with PD‑L1 tumour expression <1% versus placebo + chemotherapy. At year 5, OS rates were 13% and 9%, for pembrolizumab + chemotherapy, and placebo + chemotherapy, respectively. Median OS was 18.3 months for pembrolizumab + chemotherapy, versus 11.4 months for placebo + chemotherapy (HR 0.64; 95% CI: 0.51–0.79).8 Similarly, real-world data demonstrated that patients with tumour PD-L1 expression <1% had poorer long-term outcomes compared with those with tumour PD-L1 expression ≥50% receiving 1L I-O therapy + chemotherapy; at year 4, OS rates were 12% and 23% , respectively, in patients with sqamous mNSCLC, and 15% and 29% in those with non-squamous mNSCLC, respectively6
Dual Immuno-oncology Therapies for Patients with Tumour PD-L1 Expression <1%
A possible treatment option for patients with mNSCLC is dual I-O therapy such as an anti-CTLA-4 combined with anti-PD-(L)1 therapy, with or without chemotherapy. CTLA-4 signalling is important in the priming of the immune response and emergence of memory T cells, while inhibiting PD-1 signalling is particularly relevant for restoring the cytotoxic response to tumour cells.9-11 Dual I-O therapy ± chemotherapy has elicited survival benefits in patients with mNSCLC and tumour PD-L1 expression <1% in the CheckMate 9LA, CheckMate 227 Part 1b, and POSEIDON trials.10,12-14
In CheckMate 9LA, the 5-year OS rate was 22% for nivolumab + ipilimumab ± chemotherapy versus 8% for chemotherapy alone.13 Similarly, in CheckMate 227 Part 1b, the 5-year OS rate was 19% for nivolumab + ipilimumab compared with 10% for nivolumab + chemotherapy and 7% for chemotherapy. Furthermore, 6-year OS rates were 16%, 10%, and 5% for nivolumab + ipilimumab, nivolumab + chemotherapy, and chemotherapy, respectively.12
Conversely, in the POSEIDON trial, the 5-year OS rate was 6.1% for durvalumab + tremelimumab + chemotherapy compared with 6.5% for durvalumab + chemotherapy, and 4.0% for chemotherapy.15
In the CheckMate 227 and CheckMate 9LA trials, not only were responses to dual I-O therapy in patients with tumour PD-L1 expression <1% better than those to chemotherapy, but the quality of these responses were also higher, with longer median durations of response (DOR) versus chemotherapy. Paz-Ares highlighted the 5-year DOR rates, which demonstrate the number of patients who still respond to treatment after 5 years and show that they can achieve long-term survival with dual I-O therapy.12-14
In CheckMate 9LA, the 5-year DOR rate was 25% for nivolumab + ipilimumab + chemotherapy compared with 0% for chemotherapy.13
Similarly, in CheckMate 227 Part 1b, the 5-year DOR rate was 25% for nivolumab + ipilimumab versus 3% for chemotherapy.12
Long-term efficacy benefits were also seen in other difficult-to-treat populations treated with dual I-O therapy-based regimens, such as those with squamous histology,13,15,16 baseline brain metastases,15,17 or STK11 mutations.15,17 Paz-Ares ended the session with a reminder that there is an increased risk of treatment-related adverse events (AE) with all I-O therapies, with approximately double the rate of treatment discontinuation with dual I-O combination therapy versus chemotherapy.6,12,15,18 However, he highlighted that dual I-O combination therapies such as nivolumab + ipilimumab ± chemotherapy and durvalumab + tremelimumab + chemotherapy are viable treatment options for those with low or no tumour PD-L1 expression.5
Kicking Off Targeted Medicine for Patients with Metastatic KRASG12C NSCLC
Naidoo built on Conneran’s perspective on KRAS-mutated NSCLC to present complementary scientific data on treatment options for this previously ‘undruggable’ cancer. She introduced KRAS mutations, detailing that approximately 44% of patients with KRAS-mutated NSCLC have KRASG12C mutations,19 and that KRAS proteins have historically been undruggable due to the lack of available binding sites for small molecule inhibitors.20 Discovery of the switch II binding pocket led to the development of KRASG12C-selective inhibitors that trigger tumour cell death.21
KRASG12C Inhibitors
There have now been two Phase III trials in patients with previously treated advanced or metastatic KRASG12C NSCLC that have led to the accelerated or conditional approval of KRASG12C inhibitors adagrasib and sotorasib, in the USA, UK, and EU. Adagrasib was investigated in the randomised KRYSTAL-12 trial, while sotorasib was investigated in the CodeBreaK 200 trial. In both trials, the comparator arm was docetaxel and the primary endpoint PFS.22,23
In KRYSTAL-12, adagrasib elicited a significant improvement in median PFS: 5.5 months versus 3.8 months for docetaxel (HR: 0.58; 95% CI: 0.45–0.76; p<0.0001) and significant improvements in overall response rate (ORR; a key secondary endpoint). Improvements in median DOR, disease control rate (DCR), and intracranial ORR were also observed with adagrasib compared with docetaxel. In terms of safety, TRAEs occurred in 94% of patients receiving adagrasib compared with 86% of patients receiving docetaxel, with 48% of patients experiencing TRAEs leading to dose reduction and 59% experiencing TRAEs leading to dose interruption in the adagrasib arm. The majority of TRAEs observed with adagrasib were of a low grade in severity, and permanent discontinuation was relatively low at 8% compared with 14% for docetaxel.22
Naidoo commented that there is a learning curve in terms of how KRASG12C inhibitors are administered in clinical practice.5 In CodeBreaK 200, treatment with sotorasib also led to a significant improvement in mPFS: 5.6 months versus 4.5 months for docetaxel (HR: 0.66; 95% CI: 0.51ؘ–0.86; p=0.002), as well as numerical improvements in ORR, median DOR, DCR, and intracranial ORR. TRAEs occurred in 70% of patients receiving sotorasib compared with 86% in those receiving docetaxel,23,24 and while there have been some regulatory concerns from the US Food and Drug Administration (FDA) regarding sotorasib, both adagrasib and sotorasib are broadly available in the USA.25
There has been an increase in the number of KRASG12C inhibitors being investigated in Phase I and II clinical trials as monotherapy in the second-line setting, including olomorasib, gasorasib, glecirasib, fulzerasib, and divarasib.26
KRASG12C Inhibitors in Combination with Immuno-oncology Therapy
In the 1L setting, early-phase data on KRASG12C inhibitor + I-O therapy are available. In the Phase II part of the KRYSTAL-7 trial, adagrasib + pembrolizumab treatment was associated with a 63% ORR and a DCR of 84% in patients with tumour PD-L1 expression ≥50%. Based on these data, the Phase III part of this trial was initiated.27,28 In the Phase I CodeBreaK 101 trial, concurrent or lead-in treatment with sotorasib + atezolizumab or pembrolizumab was associated with an ORR of 29% and an mOS of 15.7 months, which, alongside other data concerning hepatotoxicity, led to the conclusion that sotorasib + I-O therapy was not a viable combination.29,30 While olomorasib + pembrolizumab is now under investigation in the Phase III SUNRAY-01 trial, in the initial Phase I LOXO-RAS-20001 trial, treatment with the combination therapy was associated with an unconfirmed ORR of 77% and DCR of 88%.31 In the Phase I MK-1084-001 trial, treatment with MK-1084 + pembrolizumab was associated with an ORR of 71% and DCR of 86% in patients with tumour PD-L1 expression ≥1%.32 It is now under investigation in a Phase III study for patients with tumour PD-L1 expression ≥50%.
In the Phase II KRYSTAL-17 trial, which is currently ongoing, adagrasib + pembrolizumab + platinum-doublet chemotherapy and maintenance adagrasib + pembrolizumab in patients who have previously received four cycles of pembrolizumab + platinum-doublet chemotherapy are being investigated.33 Other treatment combinations have also been investigated: sotorasib + chemotherapy in the Phase I CodeBreaK 101 and Phase II SCARLET trials,34,35 and fulzerasib + cetuximab in the Phase II KROCUS trial.36 Selected ongoing Phase III trials of KRASG12C inhibitors in NSCLC and the potential future directions of KRAS-directed therapy are summarised in Figure 2.
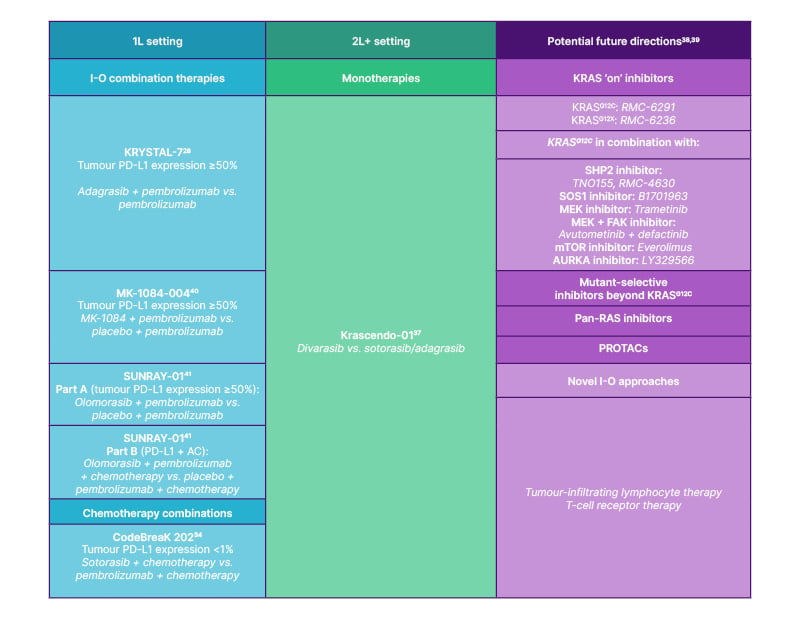
Figure 2. Summary of ongoing Phase III trials of KRASG12C inhibitors in KRASG12C NSCLC and future directions for KRAS-directed therapy.
1L: first-line; 2L: second-line; AC: adenocarcinoma; AURKA: Aurora kinase A; FAK: focal adhesion kinase; I-O: immuno-oncology; MEK: mitogen-activated protein kinase kinase; mTOR: mammalian target of rapamycin; NSCLC: non-small cell lung cancer; PD-L1: programmed death-ligand 1; PROTAC: proteolysis targeting chimera; SHP2: SH2 domain-containing tyrosine phosphatase 2; SOS1: son of sevenless homologue 1; vs.: versus.
Next-Generation Tyrosine Kinase Inhibitors: The Evolving Treatment Landscape for Cancers with ROS1 Or NTRK Fusions
Drilon introduced ROS1 fusions, which are found in up to 2% of NSCLC cases in the form of numerous fusion partners. Drilon emphasised that it is important to keep in mind that ROS1 and TRK TKIs are generational.5
ROS1 Tyrosine Kinase Inhibitors
As early-generation ROS1 TKIs, crizotinib treatment was associated with a median PFS of 19.3 months and an ORR of 72% in the PROFILE 1001 trial, while entrectinib treatment was associated with a median PFS of 15.7 months and ORR of 67.4% in the pooled ALKA-372-001, STARTRK-1, and STARTRK-2 trials.41-44 Drilon proceeded to talk about next-generation TKIs, which he defined as treatments designed for use after the failure of early-generation drugs.5 Next-generation TKIs include repotrectinib, which was associated with a median PFS of 35.7 months in the TRIDENT-1 trial (TKI-naïve patients).45 In the TRUST-I and TRUST-II trials, median PFS was not reached for taletrectinib. Drilon also highlighted the notable intracranial activity of next-generation ROS1 TKIs.46-48
Resistance to ROS1 Tyrosine Kinase Inhibitors
The ROS1 G2032R mutation was highlighted as the most relevant and commonly occurring mechanism of resistance to early generation ROS1 TKIs.49 As such, next-generation ROS1 TKIs, such as repotrectinib, taletrectinib, and zidesamtinib, have been developed to overcome resistance. In the TRIDENT-1 trial cohort consisting of patients with NSCLC who previously received a ROS1 TKI, 59% of patients with ROS1 G2032R mutations responded to treatment with repotrectinib.50 Similarly, in patients with NSCLC who previously received crizotinib in TRUST-I and TRUST-II, 67% of patients with ROS1 G2032R mutations responded to treatment with taletrectinib.46 With zidesamtinib, responses were reported in 78% of patients with ROS1 G2032R mutations who were previously treated with TKIs for NSCLC.51 Responses were also described in patients without ROS1 G2032R mutations for all three agents.46,50,51
TRK Tyrosine Kinase Inhibitors
Drilon then presented on TRK TKIs, which are tumour agnostic (Figure 3). The first TRK TKI to receive approval was larotrectinib, which elicited a median DOR of 43.3 months in patients with NTRK fusion-positive solid tumours in the pooled NAVIGATE, LOVO-TKR-14001, and SCOUT trials.52 Entrectinib was also discussed as a TRK TKI for NTRK fusion-positive solid tumours, providing a median DOR of 20.0 months.53 Next-generation TRK TKIs, such as repotrectinib, were designed to overcome mechanisms of resistance to early-generation TRK TKIs, such as G595R mutations.50
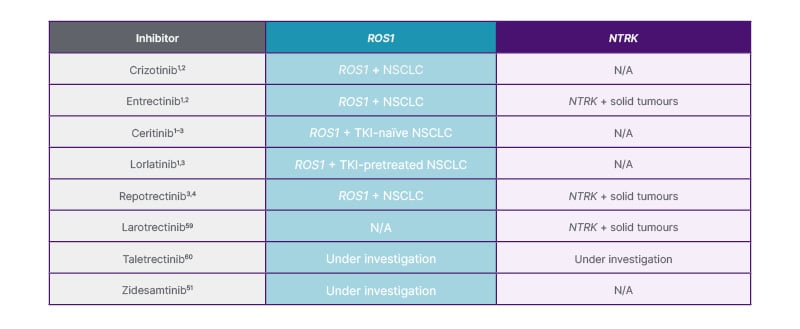
Figure 3: Overview of ROS1 and NTRK TKIs that are approved and recommended or in development.
N/A: not applicable; NSCLC: non-small cell lung cancer; NTRK: neurotrophic tyrosine receptor kinase; ROS1: proto-oncogene 1, receptor tyrosine kinase; TKI: tyrosine kinase inhibitor.
Data of repotrectinib in TKI-naive and TKI-pretreated tumours were presented, showing a 12-month DOR of 86% and 39%, respectively.54 Drilon closed this session by providing an overview of the safety profiles of ROS1 and TRK TKIs. Treatment with entrectinib and next-generation TKIs such as repotrectinib and taletrectinib may be associated with a risk of experiencing neurological adverse events.42,46,48,55-59 Drilon emphasised the importance of discussing the possible side effects of these treatments with patients in clinical practice.
Panel Discussion: What Would Your Treatment Decision Be?
In an interactive session, the speakers came together to discuss three patient cases, and asked the audience how they would approach treatment in a range of different scenarios. The first case, presented by Paz-Ares, focused on I-O treatment options, and the audience was asked to select which I-O therapy they would choose for various scenarios, such as a patient with Stage IV non-squamous NSCLC with tumour PD-L1 <1% and with or without brain metastases.
The second case, presented by Naidoo, focused on treatment options for patients with KRASG12C mutations and tumour PD-L1 expression <1%. Questions for the audience included whether they would choose standard-of-care I-O + chemotherapy or enrolment in a clinical trial of the treatment options discussed in Naidoo’s presentation earlier for a patient that was treatment-naïve. The final patient case was presented by Drilon and the audience was asked to select treatment options for patients with tumour PD-L1 expression >50% and genomic alterations such as ROS1, as discussed in his presentation.
Before the end of the symposium, the panel answered questions submitted by the audience, across a range of topics including the importance of networking to learn about rare gene-related cancers, treatment options for patients with tumour PD-L1 expression <1%, and how patient support can vary between countries, with a particular focus on supporting those living in areas with low literacy levels.







