Meeting Summary
This industry symposium took place during the 2023 European Society for Medical Oncology (ESMO) Congress in Madrid, Spain, with a goal of presenting the latest recommendations and upcoming treatment strategies for patients with oestrogen-receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer, who experience disease progression after cyclin-dependent kinase 4/6 (CDK4/6) inhibitors.
An expert panel of clinicians explained that most patients will eventually develop resistance to endocrine therapy during the metastatic setting, and there remains a considerable margin for improvement in the second-line (2L) treatment of these patients. Data for current therapeutic options in this patient population were presented, showing that patients who have previously received CDK4/6 inhibitor therapy are often resistant to many of the available 2L therapies, including combination therapies, and that resistance appears during first-line (1L) treatment, becoming particularly significant in tumours harbouring ERS1 mutations.
The recent approval of the oral selective oestrogen receptor degrader (SERD), elacestrant, was also discussed. The supporting data for this monotherapy at 2L was presented, along with changes made to the U.S. Food and Drug Administration (FDA) and European Union (EU) best practice recommendations to accommodate this therapeutic option. The panel stressed the importance of testing for ESR1 mutations at each progression during the metastatic treatment course, which is particularly relevant following the approval of elacestrant, for which ESR1 mutation is a predictive factor for efficacy.
Introduction
Over 70% of breast cancers are ER+/HER2-, for which the backbone of treatment is endocrine therapy.1-3 Endocrine therapies for ER+/HER2- advanced breast cancer at 1L include aromatase inhibitors, such as anastrozole, letrozole, and exemestane; selective oestrogen receptor modulators, such as tamoxifen; and SERDs, such as fulvestrant.4
Antonio Llombart-Cussac, Head of Medical Oncology at the Arnau de Vilanova Hospital in Valencia, Spain, emphasised that the addition of CDK4/6 inhibitors such as palbociclib, ribociclib, and abemaciclib to endocrine therapy represented a step-change in the treatment of ER+/HER2- breast cancer. CDK4/6 inhibitors provided benefits both in terms of progression-free survival (PFS) and overall survival (OS), through the suppression of cell proliferation.3-6 Median duration of treatment with endocrine therapies plus CDK4/6 inhibitors is 15–24 months.7-10 This drug combination is the current standard of care (SoC) 1L therapy for patients with recurrent, unresectable, or advanced ER+/HER2- breast cancer who are not at risk of imminent organ failure.1,11,12
However, Llombart-Cussac stressed that most patients will eventually develop resistance to endocrine therapy, and there remains a considerable margin for improvement in the 2L treatment of these patients.11
Resistance to endocrine therapy can be defined in terms of primary versus secondary clinical resistance, and de novo versus acquired molecular resistance.11 In the metastatic setting, primary resistance is defined as disease progression within the first 6 months of 1L endocrine therapy for advanced breast cancer, while secondary resistance is characterised as disease progression ≥6 months after initiating endocrine therapy in the same setting.11,13,14 Examples of de novo resistance include alterations to the PI3K/AKT/mTOR pathway, RB1 downregulation, or TP53 activation. Acquired resistance mechanisms occur after prior endocrine therapy in the metastatic setting.14,15
A key mechanism of resistance to endocrine therapy is the emergence, during the treatment in the metastatic setting, of mutations in the ESR1 gene, which encodes the oestrogen receptor.16 Mutations that alter the ligand-binding domain of the oestrogen receptor can result in constitutive activation of the receptor and therefore oestrogen independence.15,17 Llombart-Cussac noted that ESR1 mutations can affect up to 40% of patients with ER+ advanced or metastatic breast cancer who have received endocrine therapy during 1L treatment.18
The Current and Future Treatment Landscape for ER+/HER2- Advanced Breast Cancer
Virginia Kaklamani
Best practice guidelines in Europe and the USA recommend that patients with ER+/HER2- advanced breast cancer who experience disease progression on CDK4/6 inhibitors plus endocrine therapy should be treated with sequential endocrine therapy options.11,12 Virginia Kaklamani, Professor of Medicine in the Division of Hematology/Oncology, and leader of the Breast Cancer Program at the UT Health San Antonio MD Anderson Cancer Center, Texas, USA, explained that there are two options for treatment at this stage. The first is to consider endocrine monotherapy with aromatase inhibitors or fulvestrant (depending on the previous therapy), and the second is to consider endocrine therapy combinations, such as the addition of CDK4/6 inhibitors, or other agents that target the PI3K/AKT/mTOR pathway, such as everolimus, alpelisib, or capivasertib.11,12
However, Kaklamani stressed that there is room for improvement in the post-CDK4/6 inhibitor treatment landscape. She explained that monotherapy treatment options at this stage have a median PFS (mPFS) of 2–4 months.19-22 Fulvestrant, administered by intramuscular injection, is associated with injection site reactions, asthenia, nausea, and increased hepatic enzymes, though Kaklamani emphasised that its toxicity profile is generally favourable.23 Combination therapies with everolimus, alpelisib, or capivasertib can introduce additional adverse effects, such as rash, stomatitis, diarrhoea, and hyperglycaemia.24,25 If an oncologist elects to re-challenge a patient with CDK4/6 inhibitors, then adverse effects, such as myelosuppression and diarrhoea, may occur.8,26,27 Discontinuation rates, particularly with PI3K/AKT/mTOR inhibitors, can be considerable, reaching up to 25%.24,25
The 2L treatment landscape for patients with ER+/HER2- advanced breast cancer is evolving.28 Each of the available treatments has a distinct mechanism of action, which is beneficial, because when one agent generates resistance in a tumour, using an agent with a different mechanism of action for subsequent therapy may overcome some of that resistance.
Efficacy of 2L+ Endocrine Therapy Regimens for ER+/HER2- Advanced Breast Cancer With No Prior CDK4/6 Inhibitor Therapy
In patients who relapsed on 1L therapy that did not include a CDK4/6 inhibitor, monotherapy with fulvestrant was associated with an mPFS of 4.8 months in the SoFEA trial (n=231; Figure 1).29 In comparison, combination therapies are associated with a longer mPFS: everolimus + exemestane had an mPFS of 11.0 months (n=485), which was superior to placebo + exemestane (hazard ratio [HR]: 0.38; p<0.0001) in the BOLERO-2 trial;30 and alpelisib + fulvestrant had an mPFS of 11.0 months (n=169), which was superior to placebo + fulvestrant (mPFS of 5.7 months; n=172), in a population with PIK3CA mutations in the SOLAR-1 trial (HR: 0.65; p<0.001).31 In the MONALEESA-3 trial, a CDK4/6 inhibitor + endocrine therapy in a population with early relapse, or receiving 2L therapy, was associated with an mPFS of 14.6 months (n=237).9
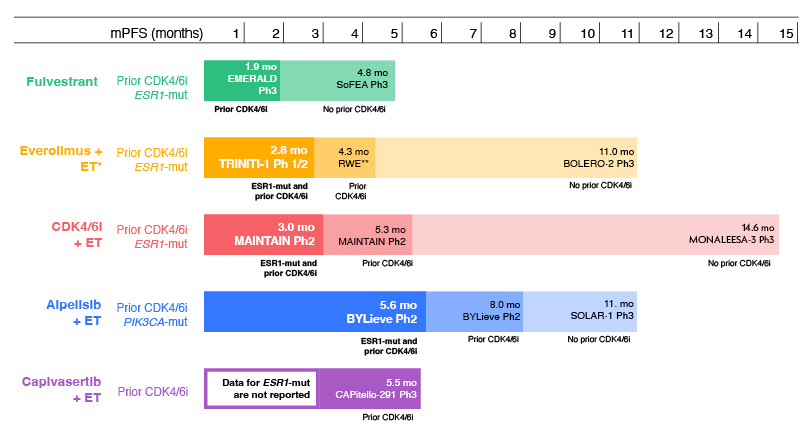
Figure 1: Efficacy of 2L+ ET regimens for ER+/HER2- advanced breast cancer with prior CDK4/6 inhibitor therapy and ESR1-mutation: indirect comparison of trial data.9,19,29-36
*Plus ribociclib.
**The RWE study of everolimus reported time to next treatment data, not mPFS.
2L: second-line; CDK4/6: cyclin-dependent kinase 4/6; CDK4/6i: CDK4/6 inhibitor; ER: oestrogen receptor; ET: endocrine therapy; HER2: human epidermal growth factor receptor 2; mo: months; mPFS: median progression-free survival; mut: mutation; RWE: real-world evidence.
Capivasertib is an inhibitor of AKT, a key protein in the PI3K/AKT/mTOR pathway. This novel agent has been approved by the FDA as a combination therapy with intramuscular fulvestrant, for patients with advanced breast cancer with one or more biomarker alterations (PIK3CA, AKT1, or PTEN).37 Kaklamani explained data from the recent CAPItello-291 trial showed that capivasertib + fulvestrant (n=155) compared with placebo + fulvestrant (n=134), was associated with a significantly improved mPFS of 7.3 months versus 3.1 months, respectively (HR: 0.50; p<0.001); 29% of included patients had no prior CDK4/6 inhibitor exposure.22
Efficacy of 2L+ Endocrine Therapy Regimens for ER+/HER2- Advanced Breast Cancer With Prior CDK4/6 Inhibitor Therapy
Kaklamani stressed that outcomes in patients who experience disease progression on therapy that included a CDK4/6 inhibitor are generally poor (Figure 1). In this population, fulvestrant monotherapy was associated with an mPFS of 1.9 months in the VERONICA trial (n=52).20 In a real-world study, combination therapy with everolimus + exemestane was associated with a time to next treatment of 4.3 months (n=622),38 and the TRINITI-1 trial reported that triple combination therapy with everolimus + exemestane + ribociclib resulted in an mPFS of 5.7 months (n=95).32 In patients with a PIK3CA mutation, mPFS with alpelisib + fulvestrant was 8.0 months in the BYLieve trial (n=127).33 In the post-CDK4/6 inhibitor population, the CAPItello-291 trial showed that capivasertib was associated with an mPFS of 5.5 months in the AKT-altered population (n=289).22
Several studies have investigated the efficacy of rechallenge with a CDK4/6 inhibitor in patients who have received prior CDK4/6 inhibitor therapy. In the MAINTAIN trial, ribociclib + endocrine therapy versus placebo + endocrine therapy was assessed in patients who had disease progression on a CDK4/6 inhibitor + endocrine therapy (86.5% with palbociclib included in the previous therapy).34 MAINTAIN reported an mPFS of 5.3 months in patients treated with ribociclib (n=60) versus 2.8 months in patients treated with placebo (n=59; HR: 0.57; p=0.006), indicating that rechallenge with a CDK4/6 inhibitor provided a benefit in this population. However, other trials failed to show a significant improvement in mPFS with CDK4/6 inhibitor rechallenge versus endocrine therapy alone, with the PACE trial reporting an HR for palbociclib + endocrine therapy after prior palbociclib + endocrine therapy of 1.11 (p=0.62; palbociclib + endocrine therapy: mPFS 4.6 months [n=111] versus endocrine monotherapy: mPFS 4.8 months [n=55]),40 and the PALMIRA trial reported an HR of 0.8 (p=0.206; palbociclib + endocrine therapy: mPFS 4.2 months [n=136] versus endocrine monotherapy: mPFS 3.6 months [n=62]).41 Adding the programmed death-ligand 1 inhibitor avelumab to palbociclib + endocrine therapy (n=54) showed a trend towards longer mPFS (compared with endocrine monotherapy [n=55]; 8.1 months versus 4.8 months, respectively) in the PACE trial, though the difference did not reach statistical significance (HR: 0.75; p=0.23).40
Efficacy of 2L+ Endocrine Therapy Regimens for ER+/HER2- Advanced Breast Cancer With No Prior CDK4/6 Inhibitor Therapy and ESR1 Mutation
Outcomes are particularly poor in patients who have received CDK4/6 inhibitor therapy and harbour an ESR1 mutation ([/hl]Figure 1). Kaklamani explained that in these patients, fulvestrant monotherapy was associated with an mPFS of 1.9 months in the EMERALD trial (n=165).19 In the MAINTAIN trial, rechallenge with a CDK4/6 inhibitor + endocrine therapy was associated with an mPFS of 3.0 months (n=18).34 Triple therapy with ribociclib + everolimus + endocrine therapy in the TRINITI-1 trial was associated with mPFS of 2.8 months (n=30).35 The BYLieve trial reported that alpelisib + fulvestrant was associated with an mPFS of 5.6 months in patients with a PIK3CA mutation (n=27).36 Capivasertib + fulvestrant data in patients with ESR1-mut tumours is not available.22
Kaklamani explained that, together, these data indicate that patients who have previously received CDK4/6 inhibitor therapy are more likely to have resistant disease to many of the available therapies, including combination therapies, than those who have not, and that resistance appears to be particularly substantial in tumours harbouring ERS1 mutations.
Oral Selective Oestrogen Receptor Down-Regulators
Oral SERDs have the potential to deliver a higher bioavailable dose than can be achieved with fulvestrant, a SERD administered through intramuscular injection. Kaklamani explained that this is an approach which is likely to be required for the successful treatment of patients with ESR1 mutations. One oral SERD, elacestrant, has recently been approved as a 2L monotherapy for advanced breast cancer in the USA and EU.42,43
Phase II trials of oral SERDs, giredestrant, and amcenestrant, failed to show a significant PFS benefit at 2L in patients with ERS1 mutations.44,45 However, a Phase II trial of camizestrant versus fulvestrant demonstrated a trend towards improved mPFS in patients with an ESR1 mutation (camizestrant 75 mg [n=22] versus fulvestrant 500 mg [n=35]: 6.3 months versus 2.2 months; HR: 0.33). Of note, only 50% of the patients in this trial had prior exposure to CDK4/6 inhibitors.21
Kaklamani presented data from EMERALD, the first and only Phase III trial of an oral SERD to meet its primary endpoint.19 EMERALD enrolled patients with ER+/HER2- breast cancer who had received prior CDK4/6 inhibitor therapy, had ≤1 line of chemotherapy, and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Patients were randomised to receive either elacestrant 345 mg once daily (n=239), or the investigators’ choice of SoC endocrine therapy from fulvestrant, anastrozole, letrozole, or exemestane, depending on the prior endocrine therapy used (n=239). The two primary endpoints were PFS in patients with an ESR1 mutated tumour, and PFS in all patients. A tumour ESR1 mutation was detected in 47.8% of patients. All patients had previously received a CDK4/6 inhibitor, and enrolment of patients with primary endocrine resistant breast cancer was allowed.
Results showed that elacestrant (n=115) provided a 45% reduction in risk of progression or death versus SoC (n=113) in patients with tumours with ESR1 mutations, with an mPFS of 3.8 versus 1.9 months (HR: 0.55; p=0.0005). A closer look at the Kaplan–Meier curves reveals an initial drop in both arms, which highlights primary endocrine resistance (present in almost 25% of the EMERALD patient population), but then it also shows a sustainable separation of the curves in the more endocrine-sensitive setting. Since median PFS alone may not be enough to interpret results in this scenario, PFS landmark analyses were conducted at 6 and 12 months.19 The 6-month PFS rate reported in the EMERALD trial was 40.8% with elacestrant versus 19.1% with SoC, and the 12-month PFS rate was 26.8% versus 8.2%, respectively, demonstrating substantial and clinically meaningful improvements in PFS at these timepoints.
With the intention to identify tumours that remain endocrine sensitive despite an acquired resistance to aromatase inhibitors or fulvestrant, the impact of prior CDK4/6 inhibitor therapy duration for at least 6, 12, and 18 months on PFS in patients with ESR1-mutated tumours receiving elacestrant versus SoC endocrine therapy was examined. In patients with at least 6 months of prior CDK4/6 inhibitor duration, median PFS with elacestrant was 4.1 versus 1.9 months with SoC endocrine therapy (HR: 0.52). In patients with at least 12 months prior CDK4/6 inhibitor duration, median PFS with elacestrant increased to 8.6 versus 1.9 months with SoC (HR: 0.41).46 Results for patients with at least 18 months of CDK4/6 inhibitor duration are similar. These data indicate that elacestrant achieves the greatest benefit in patients with relative endocrine sensitivity leading to clinically meaningful benefit versus SoC.
Elacestrant was well tolerated, with most adverse events (AE), including nausea, at Grade I or II in severity.46 The discontinuation rate due to an AE was 3.4% in the elacestrant arm and 0.9% in the SoC arm.46 The most common AEs were musculoskeletal pain, nausea, fatigue, and vomiting.19 Grade III nausea was reported in 2.5% of patients treated with elacestrant versus 0.9% of patients treated with SoC.19 A low percentage of patients received an antiemetic (8.0% with elacestrant, 10.3% with an aromatase inhibitor, and 3.7% with fulvestrant).46
Kaklamani concluded by emphasising the importance of delivering personalised care for patients with ER+/HER2- advanced breast cancer. When selecting 2L therapy, the relative benefit of combinations versus monotherapy and the increased prevalence of AEs with combination therapies must be considered.12 Since data suggest that greater PFS benefit is achieved with targeted agents than SoC in biomarker-selected patient subgroups,19,22,39 a biomarker-driven treatment algorithm is needed to ensure optimal treatment selection, especially for those patients whose tumours remain endocrine-sensitive (CDK4/6 inhibitor duration for at least 12 months).12,22,19,36
Emerging Biomarkers in Breast Cancer: Implementing Liquid Biopsy ESR1 Mutation Testing
Volkmar Müller
Volkmar Müller, Professor of Medicine and Deputy Director at the Department of Obstetrics and University Breast Centre, University Hospital Hamburg-Eppendorf (UKE), Hamburg, Germany, explained that the significance of ESR1 mutations in breast cancer was not fully appreciated until 2013, when a series of studies identified ESR1 mutations in 11–55% of advanced ER+ breast cancers with prior aromatase inhibitor exposure.47
Binding of oestrogen to the oestrogen receptor leads to conformational changes in the ligand-binding domain of the receptor, resulting in ligand-dependent activation, coactivator recruitment, downstream regulation of gene expression, which is associated with cancer growth.18 Mutations in ESR1 alter the ligand-binding domain of the oestrogen receptor, resulting in a ligand-independent, constitutively active conformation that enhances cancer growth, metastasis, and resistance.18 These mutations decrease the affinity of the oestrogen receptor for oestrogen, and for endocrine therapies such as fulvestrant that target the receptor.18
Combined data from clinical trials indicate that ESR1 mutations represent a negative prognostic marker in advanced breast cancer.48 Müller explained that there was little benefit to testing for this mutation until a clear advantage could be derived from selecting specific treatment approaches for patients harbouring these mutations. However, he emphasised that the recent clinical trial data described by Kaklamani indicate that patients with an ESR1 mutation derive greater benefit from elacestrant than from SoC therapies.19 Testing for ESR1 mutations is now recommended in patients with ER+/HER2- metastatic breast cancer at progression, to ensure that they could receive appropriate treatment with elacestrant monotherapy, or another endocrine therapy either alone or in combination with targeted agents.49
Müller stressed that testing for ESR1 mutations should occur at each progression during the metastatic treatment course, unless detected previously.18-21 This is because the longer a tumour is exposed to endocrine therapy in the metastatic setting, the greater the chance of developing an ESR1 mutation (Figure 2). 18,19,50-55
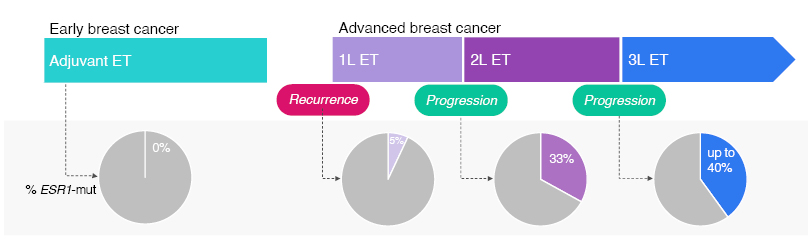
Figure 2: Longer exposure to endocrine therapy in 1L metastatic breast cancer increases the chance of developing ESR1 mutation.18,19,50-54
1L: first-line; 2L: second-line; 3L: third-line; ET: endocrine therapy; mut: mutation.
ESR1 mutations can be identified in breast cancer through either liquid or tissue biopsy. However, Müller emphasised that liquid biopsy has several advantages compared with tissue biopsy: it is minimally invasive, can be repeated regularly without inconvenience and risk for patients, has high sensitivity, rapid sample acquisition, enables real-time monitoring, and reveals tumour heterogeneity.56,57 For these reasons, blood-based circulating tumour DNA liquid biopsy is preferred owing to greater sensitivity in detecting ESR1 mutation status, as recommended by ESMO, National Comprehensive Cancer Network (NCCN), and American Society of Clinical Oncology (ASCO) guidelines.49,57,58 Next generation sequencing-based assays are widely used to detect ESR1 mutations because of their broad dynamic range, though droplet digital PCR offers high sensitivity, economic feasibility, and highly readable and repeatable results.59,60 There is a strong concordance between ESR1 mutation allele frequency detected by droplet digital PCR and next generation sequencing.54
Müller concluded that mutation of ESR1 is clinically relevant during endocrine treatment.13 ESR1 mutations are both prognostic, and predictive of response to elacestrant,13,14,57 and therefore, testing via liquid biopsy should occur at each progression during the metastatic treatment course, unless detected previously.50,51
Closing Remarks
Antonio Llombart-Cussac
Llombart-Cussac presented two hypothetical case studies to emphasise the points made during the symposium.
Case 1 was a 60-year-old post-menopausal female with an initial diagnosis of ER+/HER2- luminal A invasive duct carcinoma (T2N1M0). She was treated with a left breast lumpectomy and axillary lymph node dissection. After 5 years of remission, she presented with a persistent cough, and supraclavicular lymph node and lung metastases were detected via CT scan. Her ECOG PS was 0, and she was not in visceral crisis. During 1L treatment with endocrine therapy + CDK4/6 inhibitor, a routine follow-up CT at 24 months identified enlargement of existing lung lesions and a new 1.5 cm lesion in the left femur.
Following disease progression on endocrine therapy + CDK4/6 inhibitor, the ESMO guidelines recommend biomarker testing to guide the selection of appropriate 2L treatment for metastatic ER+/HER2- breast cancer (Figure 3).2 In this case, liquid biopsy detected an ESR1 mutation, with wild-type PIK3CA and BRCA1/2, and elacestrant was selected as 2L therapy.
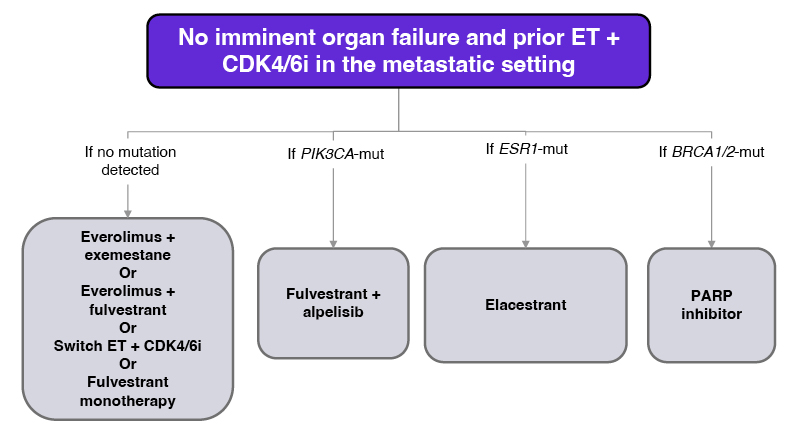
Figure 3: ESMO metastatic breast cancer treatment guideline.2
CDK4/6i: cyclin-dependent kinase 4/6 inhibitor; ER: oestrogen receptor; ESMO: European Society for Medical Oncology; ET: endocrine therapy; mut: mutation; PARP: poly (ADP-ribose) polymerase.
Case 2 was a 57-year-old, post-menopausal female with de novo ER+/HER2- luminal A invasive ductal carcinoma (T2N1M1). She had bone and liver metastases, with normal function and no organ failure at diagnosis, and an ECOG PS of 1. The patient was treated with abemaciclib and anastrozole at 1L. After 16 months, following reports of Grade II asthenia and back pain, a CT scan showed enlargement of existing liver lesions, with normal liver function, and two additional bone lesions.
Liquid biopsy revealed ESR1 and PIK3CA co-mutations. With no imminent organ failure, treatment recommendations for this patient include fulvestrant + alpelisib, and elacestrant.2 Llombart-Cussac noted that in a case like this one, it is important to consider factors such as the duration of exposure to CDK4/6 inhibitor therapy, which is indicative of a remaining endocrine sensitivity. He also pointed out that PIK3CA mutations are associated with de novo resistance to endocrine therapy, occurring prior to or early in endocrine therapy,11,14,62 whereas ESR1 mutations are a form of acquired resistance to endocrine therapy, which generally occur following exposure to endocrine therapy in the metastatic setting, often become the driver of the disease, and require a targeted therapy.11,18,57,62
Llombart-Cussac concluded the symposium with six key points:
• CDK4/6 inhibitors are the SoC in 1L treatment of ER+/HER2- advanced breast cancer.4,5,12
• 2L treatment options include sequential endocrine monotherapies or combination therapies until exhaustion of endocrine-therapy-based regimens.11,16
• Guidelines strongly recommend that 2L treatment decisions are based on mutation status.2,49,58
• Longer prior duration of CDK4/6 inhibition in patients with ESR1 mutations is a predictive factor for response to elacestrant.18,19,61
• In the presence of coexisting mutations, both PIK3CA and ESR1, data suggest that the endocrine pathway may be the driver of disease.32,35
• ESR1 mutation testing is essential at each progression during the metastatic treatment course, to identify patients who are most likely to respond to targeted treatments.18,50-52







