Meeting Summary
This year’s European Organisation for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Tumours Group (CLTG) Annual Meeting was held in Madrid, Spain. Presentations covered a broad range of topics in the field of cutaneous lymphomas, from molecular mechanisms, through diagnostics and prediction of prognosis, to skin-directed and targeted therapies, combination therapies, and patient-reported outcomes.
There was an overall feeling at the EORTC-CLTG meeting that further research is needed to better define the molecular basis for the different forms of cutaneous T cell lymphoma (CTCL), which will facilitate the development of novel treatments and therapeutic drug combinations. However, it was clear that significant progress had been made in understanding the biology behind CTCL, and that a broad range of therapies are available that provide symptomatic control in many patients.
Authors: Nicola Humphry
Affiliation: Nottingham, UK
Disclosure: Humphry has declared no conflicts of interest.
Support: The publication of this article was funded by Helsinn Healthcare SA.
Citation: EMJ Oncol. 2022;10[Suppl 11]:2–11. DOI/10.33590/emjoncol/10045588. https://doi.org/10.33590/emjoncol/10045588.
Introduction
Cutaneous T cell lymphoma (CTCL) is the predominant form of primary cutaneous lymphoma and comprises a heterogenous group of rare diseases.1 Mycosis fungoides (MF) is the most common CTCL, accounting for 62% of cases, while other CTCL types such as Sézary syndrome (SS) are less prevalent.1
MF is caused by the malignant transformation of effector memory T cells in the skin.2 MF is usually associated with an indolent clinical course, and can remain stable for many years, though painful, itchy lesions can affect quality of life.2,3 Ulcerating tumours may occur in more advanced disease.4 SS is an erythrodermic, leukaemic variant of CTCL, thought to arise from thymic memory T cells, which is characterised by blood involvement and lymphadenopathy.2,4
Molecular Mechanisms in Cutaneous T Cell Lymphoma
Martine Bagot and Manuel Serrano
Bagot (Hôpital Saint Louis, Paris, France) presented the current understanding of the tumour microenvironment (TME) in MF and SS as part of an industry-sponsored symposium. It was explained that in both of these forms of CTCL, malignant T cells cause a shift from a predominantly Type 1 helper T cell to a Type 2 helper T cell immune profile in the TME.5,6 Progressive immune dysfunction is associated with tumour cell growth, with a shift from anti-tumorigenic tumour-associated macrophages to pro-tumorigenic tumour-associated macrophages.7 It was emphasised that optimal therapies for MF and SS are likely to be those targeting malignant cells and/or restoring the Type 1 helper T cell/Type 2 helper T cell balance.5-7
Cellular senescence plays a role in post-therapeutic outcomes in CTCL and other malignancies. Research data presented by Serrano (Institute for Research in Biomedicine, Barcelona, Spain) during the symposium explained that senescent cells are often able to avoid cancer immunotherapy by secreting immunosuppressive factors, and that, upon cessation of therapy, this environment permits the regrowth of tumour cells.8 It was emphasised that senolytic drugs, such as those that target programmed death ligand 2, can be used to induce apoptosis in senescent cells, and that promising pre-clinical data have been published regarding senolytic drugs, including when used in combination with other agents such as immune checkpoint inhibitors.9-12
Diagnosis of Cutaneous T Cell Lymphoma
Miguel Piris, Thom Doeleman, Ruth Alonso-Alonso, and Pietro Quaglino
Data presented by Piris (Fundación Jiménez Diaz, Madrid, Spain) as part of another industry-sponsored symposium indicated that the application of immunohistochemistry in CTCL diagnosis has reached a plateau, and that molecular diagnostics should be at the centre of routine diagnostics. To facilitate the introduction of new, non-cytotoxic therapies, research needs to better define the molecular bases of the different forms of CTCL, and mechanistic models are needed to analyse the mutated genes and pathways involved. Furthermore, Piris explained that more information is needed regarding the contribution of the stroma, the role of potential infectious agents, the mutations present in lymphoid progenitor cells, and sub-clonal dynamics in CTCL.
The application of artificial intelligence (AI) could potentially help to distinguish between early MF and benign inflammatory dermatoses. Research data from Doeleman (Leiden University Medical Center, the Netherlands), described a proof-of-concept study that evaluated the use of deep learning to assist with the classification of these conditions using digital skin biopsies.23 An institutional dataset of images from patients with MF (n=178) or inflammatory dermatoses (n=165) was used. At a magnification of 200x, the trained AI algorithm differentiated MF from inflammatory dermatoses with a similar level of accuracy as two expert pathologists. Doeleman explained that although these results indicate the potential of AI to support MF diagnostics, this model requires expansion into a multi-institutional dataset.
Evaluating differential gene expression during the progression of CTCL may be one approach to improving staging. Alonso-Alonso (Instituto de Investigación Sanitaria, Hospital Universitario Fundación Jiménez Diaz, Madrid, Spain) presented data from a NanoString (NanoString Technologies, Seattle, Washington, USA) analysis of tissue samples from patients with MF (n=31) or inflammatory dermatoses (n=16) at a single centre in Spain.14 Unsupervised gene clustering was used to analyse the differential expression of a custom panel of genes. Differential expression of several genes was identified in patients with MF when compared to those with inflammatory dermatoses, and differences were also observed between early MF and advanced MF. It was noted that the high degree of inter-tumoural heterogeneity observed in this study suggests the existence of individual molecular signature features in patients.
Staging of MF includes the identification of patches, plaques, and tumours on the skin of the patient, although differentiating between these lesion types can be rather subjective.15 The current tumour, node, metastasis plus blood staging system does not modify MF stage according to the presence of plaques,15 despite their association with lower overall survival (OS) and progression-free survival (PFS),16,17 and with more frequent use of systemic therapy at first-line.18 Quaglino (Università degli Studi di Torino, Turin, Italy) presented data from a survey of expert physicians in which all 22 respondents concurred that it was important to distinguish between patches and plaques in early-stage MF, and over half agreed that a biopsy should be performed for confirmation.19 Most respondents felt that the current positioning of plaques in the tumour, node, metastasis plus blood system was clinically inadequate. Quaglino concluded that prospective studies are needed to identify clinicopathological correlations between prognosis and patches/plaques and emphasised that there is a role for biomarkers in distinguishing disease stage.
Prediction of Prognosis
Julia Scarisbrick and Vanessa Gargallo Moneva
The estimated 5-year survival rate ranges from 89% to 98% for early-stage MF, and prognostic risk factors include advanced disease stage and older age.16 Additionally, higher levels of β2-microglobulin, lactate dehydrogenase, and total IgE are associated with a more advanced disease stage.20
Scarisbrick (University Hospital Birmingham and the University of Birmingham, UK) reported the results of an analysis of the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study, which aimed to identify prognostic factors in MF and SS (n=698).21 In early-stage CTCL, the presence of plaques and large cell transformation (LCT) in the skin were associated with disease progression. Characteristics that differed between patients with late-stage versus early-stage disease included older age, poorer quality of life, and greater disease-related mortality. A prognostic index consisting of four risk factors (Stage IV disease, age >60 years, raised lactate dehydrogenase level, and LCT in the skin) permitted the stratification of patients into low-, intermediate-, and high-risk groups, with significant differences in the rates of disease progression and OS between groups.
A retrospective genetic analysis of biopsy samples from 51 patients with early-stage MF, of whom 21 experienced disease progression over a median follow-up of 8 years, was presented by Gargallo Moneva (Hospital Universitario del Sureste, Arganda del Rey, Madrid, Spain).22 Samples were analysed using NanoString technology by using a panel of 97 genes. Several genes were highly expressed in the no-progression group versus the progression group, including genes encoding cluster of differentiation (CD) 8, which has been associated with better prognosis in MF,23,24 and CXCL10, which is related to epidermotropism.25,26 On the other hand, genes that were more highly expressed in the progression group versus the no-progression group included CD79A/B, CD19, and CXCL13. Interestingly, CD79A/B and CD19 are associated with B cells, which are found in greater numbers in more advanced MF,27 and CXCL13 encodes B cell-attracting chemokine 1, which acts as a B cell chemoattractant.28
Updates in Cutaneous T Cell Lymphoma Treatment
Pablo Ortiz-Romero, Emanuella Guenova, Chalid Assafj, Julia Scarisbrick, Keila Mitsunaga, Hélène Moins-Teisserenc, Janika Gosmann, Evangelina Papadavid, Maxime Battistella, Christina Mitteldorf, Mathias Oymanns, and Laura Gleason
Topical therapies, phototherapy, local radiation therapy, and total skin electron-beam therapy (TSEBT) play important roles in the treatment of early-stage MF, when symptoms are generally restricted to inflammatory lesions on the skin (Figure 1).29,30 Advanced-stage MF is more likely to be managed with systemic therapies, with guideline-recommended options such as brentuximab vedotin (brentuximab), bexarotene, extracorporeal photopheresis (ECP), interferon (IFN), and methotrexate, among others.29 Updates on several of these therapeutic approaches were presented at the EORTC-CLTG 2022 meeting, as follows.
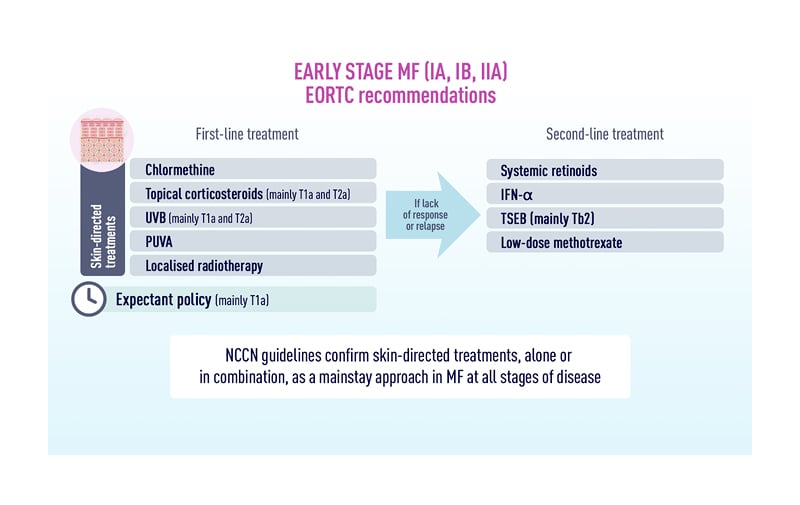
Figure 1: Recommendations for first- and second-line treatment of early-stage mycosis fungoides.
IFN: interferon; MF: mycosis fungoides; NCCN: National Comprehensive Cancer Network; PUVA: photodynamic therapy; TSEB: total skin electron beam therapy; UVB: ultraviolet B. Reproduced with permission from MF Talks.26
Chlormethine Gel
Chlormethine (also known as mechlorethamine) is an alkylating agent that predominantly inhibits rapidly proliferating malignant T cells.32,33 Chlormethine has been used as a treatment for patients with MF since the 1950s, and a gel formulation was more recently approved in several countries, including the European Union (EU) and USA.33–35
In an industry-sponsored symposium, Ortiz-Romero (Hospital Universitario 12 de Octubre, Madrid, Spain), presented data from a recent in vitro study comparing the release rate profiles and permeation characteristics of chlormethine gel and ointment.36 The results indicated that chlormethine release over a 5-hour period was significantly higher for the gel than for the ointment and that minimal amounts of chlormethine passed through the epidermal tissue to reach the dermal tissue. Furthermore, Ortiz-Romero emphasised that bioanalytical assays have shown a lack of evidence for systemic absorption of topical chlormethine gel.37
Another in vitro study assessed the effects of chlormethine gel on malignant T cells in the skin.32 During the same symposium, Guenova (University Hospital of Lausanne, University of Lausanne, Switzerland) explained that chlormethine decreased T cell viability in a time- and dose-dependent manner, predominantly through the induction of DNA double-stranded breaks. In addition, chlormethine induced the expression of the pro-apoptotic CASP3 gene and down-regulated genes related to DNA repair.32
One of the pivotal trials of chlormethine gel was the randomised, controlled, observer-blinded, multicentre 201 trial, which showed that, in terms of response rate, the gel formulation was non-inferior to the ointment in patients with early-stage MF (n=260).38 Assaf (Helios Klinikum Krefeld, Lutherplatz, Germany), described a recent post hoc analysis of these data, which aimed to evaluate whether prior exposure to bexarotene or phototherapy affected the response to chlormethine gel (n=118).39 The results showed no statistically significant difference in the time to first response (measured by the Composite Assessment of Index Lesion Severity [CAILS]) between patients who had received prior bexarotene and/or phototherapy and those who had not, indicating that chlormethine is a valid treatment option in patients who have received prior therapy for MF.
Alberti-Violetti (University of Milan, Milan, Italy), presented a poster describing the results of a retrospective, multicentre, observational study which aimed to evaluate the efficacy and tolerability of chlormethine gel in a real-world Italian cohort.40 A total of 68 patients with MF were included, of which 63% were male and the majority had classical MF (76%) and Stage I disease (68%). Chlormethine gel was prescribed either as monotherapy or in combination with a systemic treatment. Two-thirds of patients applied chlormethine gel at a frequency of 4–5 days per week, with one-third applying the gel on a daily basis. Over 3/4 of patients achieved a complete or partial response (as measured by the Severity Weighted Assessment Tool [mSWAT]). No patient experienced a severe adverse event (AE). Hyperpigmentation and irritant contact dermatitis were the most common AEs, each occurring in more than half of patients. Alberti-Violetti concluded that this study confirms the efficacy and tolerability of chlormethine gel for MF in clinical practice in Italy.
Extracorporeal Photopheresis
In ECP, a patient’s blood is exposed extracorporeally to photoactivated 8-methoxypsoralen.41 In one industry-sponsored symposium, Scarisbrick explained that guidelines recommend ECP as a first-line treatment option for Stage III MF and for SS,30,41,42 and some suggest that it may be suitable for earlier stages of MF with blood involvement.30 ECP can be safely combined with many other systemic treatments available for the treatment of CTCL, such as phototherapy, IFN, retinoids, or bexarotene, and with skin-directed therapies such as chlormethine gel.30,41,42
During the same symposium, Ortiz-Romero reported data from a Spanish real-world study of ECP in the management of CTCL (n=64). Most patients had Stage IVA1 disease at baseline (51.6%), and the median number of prior lines of systemic therapy was two. The majority of patients (82.8%) were treated with two cycles of ECP per month, and 75.0% of patients received ECP as part of a combination therapy. The overall response rate (ORR) in the skin was 49.0%, with a median duration of response of 28 weeks, and the responses to combined therapy tended to be better than those to monotherapy. Ortiz-Romero concluded that ECP is effective in a real-world setting in Spain.43
Pegylated Interferon-α
IFNα-2a has been approved for use in CTCL for over 30 years, and pegylated IFNα-2a was approved more recently, in 2002.44 However, Mitsunaga (Hospital Universitario 12 de Octubre) stressed that IFNα-2a is no longer produced in either the EU or USA, leaving pegylated IFNα-2a as the only available form of the drug for therapeutic use.45 Mitsunaga explained that conjugation to polyethylene glycol (i.e., pegylation) extends the half-life of IFNα and may reduce the frequency of acute AEs such as fatigue and flu-like symptoms, as well as neuropsychological AEs such as depression.46-48
Mitsunaga presented data from a real-world study of pegylated IFNα-2a in the treatment of primary CTCL.45 The international, multicentre, retrospective study included patients with MF or SS of any stage (n=105), and more than half the patients received pegylated IFNα-2a as part of combination therapy with ECP, bexarotene, chlormethine gel, or phototherapy. Approximately 50% of patients responded to therapy, and more than 10% of patients achieved a complete response (CR). Two-thirds of patients experienced an AE, most commonly flu-like symptoms, lymphopenia, and neutropenia.
Mogamulizumab
Mogamulizumab is an antibody that selectively binds to C-C chemokine receptor Type 4, which is expressed on the surface of malignant T cells in MF and SS, resulting in cell death. Mogamulizumab is approved in the EU for the treatment of adults with MF and SS who have received at least one prior systemic therapy.49
In an industry-sponsored symposium, Moins-Teisserenc (Université de Paris, Institut de Recherche Saint Louis, Paris, France) presented data from a prospective longitudinal study of the impact of mogamulizumab on the TME in patients with relapsed or refractory SS (n=260) or age-matched controls (n=15).50 Mogamulizumab induced early changes in the blood suggestive of immune restoration, with 65.4% of patients achieving an initial CR. Benign T cells and activated regulatory T cell levels decreased over the first month of treatment, while in the long-term, the patients experienced a sustained increase in naïve and stem memory CD4+ T cells.51
The effect of mogamulizumab on the malignant T cell population was also studied in a retrospective flow cytometry analysis of patients with MF or SS (n=11). Gosmann (University of Bochum, Germany), reported that a significant reduction in malignant T cells was observed after four cycles of treatment; substantial reductions in CD3+, CD5+/CD7-, and CD4+/CD26+ T cells were observed.52 Gosmann suggested that an increase in the malignant T cell population could be a criterion for secondary resistance, and that flow cytometry-based monitoring could lead to more personalised treatment options.
Presenting data from three recent studies, as part of one of the industry-sponsored symposia, Papadavid (National and Kapodistrian University of Athens, Greece), emphasised that real-world data support the efficacy of mogamulizumab in the clinical setting. A retrospective study of patients with advanced CTCL (n=21) in France reported a PFS of 22.0 months and an ORR of 57% over a median follow-up period of 11.6 months.51 A similar retrospective study combining patients from the UK and Spain (n=13) reported an ORR in the skin of 67% of patients. Furthermore, in patients with nodal disease, 90% achieved stable disease and 10% achieved a partial response.53 Finally, real-world data from Australia and trial data from MAVORIC were combined to compare relative survival outcomes in advanced MF or SS between mogamulizumab and vorinostat (n=182).54 This study reported that median OS was 57.2 months for mogamulizumab and 40.0 months for vorinostat, although the hazard ratio of 0.679 did not attain statistical significance (p=0.063).
Mogamulizumab can induce the development of a skin rash, termed mogamulizumab-associated rash (MAR), which has been associated with a higher response rate and longer OS.55,56 Battistella (Université Paris Cité and Hôpital Saint Louis, France) suggested that potential mechanisms to distinguish MAR from disease progression include the identification of an inverted or normalised CD4:CD8 ratio,57,58 CD8+ exocytosis,57,58 follicular involvement,57 lymphocyte atypia,55 or the retention of CD7 expression.57
Mild MAR is managed with a continuation of mogamulizumab treatment and the addition of steroids or antihistamines, while more severe MAR requires a biopsy and either delay or discontinuation of mogamulizumab.49
Mitteldorf (University Medical Center Göttingen, Germany), presented the case of a 33-year-old female patient with a novel form of MAR termed lupus miliaris disseminates faciei-like MAR.59 After 12 months of treatment, the patient developed yellowish-red papules around the eyes and nose. A biopsy revealed a dense, predominantly CD8+ infiltrate, with evidence of psoriasiform, spongiotic, folliculotropic, and granulomatous components. Therapy was discontinued, after which the rash resolved over a period of 7 weeks. It was explained that further forms of MAR are likely to be identified in the future.
Brentuximab
Brentuximab is a monoclonal antibody directed at CD30, which is fused to monomethyl auristatin E (MMAE), a potent anti-tubulin agent.60 Brentuximab is approved for the treatment of adults with CD30+ CTCL after at least one prior systemic therapy.60
Presenting evidence from four recent investigations, Papadavid explained that brentuximab outcomes in real-world studies should be interpreted with caution because of their small sample size, limited follow-up, and lack of mature data. An analysis of prospective data from the Spanish Primary Cutaneous Lymphoma Registry revealed that the ORR was 67% and the PFS was 10.3 months in patients with CTCL who were treated with brentuximab and followed up for a mean duration of 18.0 months (n=67). Furthermore, CD30 expression did not appear to influence the ORR.61 A retrospective study of patients with MF or SS, from eight European countries, showed that brentuximab achieved an overall response lasting ≥4 months in 42% of patients, an ORR of 67%, and a CR rate of 27% (n=67). This study also indicated that the efficacy of brentuximab was independent of CD30 positivity.62 A retrospective review of patients with MF and LCT in the USA (n=23) reported that the lymph node CR rate and the improvement in the modified mSWAT score were both numerically greater for brentuximab than for bexarotene, skin-directed therapy, or chemotherapy.63 Finally, a retrospective analysis of brentuximab in combination with skin-directed therapy in patients with CD30+ CTCL in Germany found that complete remission was achieved in 30.8% of patients, with an ORR of 84.6% (n=26). The authors of the analysis concluded that the addition of skin-directed therapies to brentuximab extended the time until the next therapeutic escalation.64
Combination Therapy
In addition to having cytotoxic properties, the active agent of brentuximab (MMAE) is a highly potent radiosensitiser. For example, a preclinical study showed that MMAE sensitised colorectal cancer cells and prostate cancer cells to irradiation in a schedule- and dose-dependent manner.65 Oymanns (Helios Klinikum Krefeld, Germany), presented data from a small study combining brentuximab and ultra-hypofractionated, low-dose and total skin electron-beam therapy radiation in patients with advanced stage CTCL: all four patients achieved near CR within 8 weeks of therapy.66
Gleason (Thomas Jefferson University, Philadelphia, USA), presented the results of a retrospective case series that assessed the combination of chlormethine gel and narrow-band ultraviolet B phototherapy in patients with MF (n=5).67 One patient achieved CR, three patients achieved PR, and one patient had stable disease during a treatment period of 2–11 months. Two patients experienced no AEs, and all the reported AEs were skin-related. It was concluded that combining chlormethine gel and narrow-band ultraviolet B phototherapy may be a safe and effective treatment for MF.
Perceptions of Mycosis Fungoides
Aviv Barzilai
Barzilai (Chaim Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel), described the results of an online survey of dermatologists’ perceptions of illness in early-stage MF (n=80).68,69 It was explained that dermatologists generally viewed MF as a chronic disease, which caused a moderate emotional burden but had little impact on patients’ lives. In contrast to patients’ perceptions,70 many dermatologists felt that patients were able to control their disease. The presentation emphasised that this study found that dermatologists used a high diversity of themes when presenting MF to patients, and Barzilai concluded that maintaining patient-centred communication will enable dermatologists to identify gaps between their own and their patients’ perspectives on this disease.
Closing Remarks
New research presented at the EORTC-CLTG 2022 meeting demonstrated an increasing depth of understanding of the biology behind CTCL, and the mechanisms by which a variety of therapies modify it. There was an overall view that AI may have the potential to support the diagnosis of CTCL type and stage.
Novel data were generally supportive of the efficacy and safety of available therapies in a real-world setting, although an important need still remains for newer treatments and therapeutic drug combinations. Further research is needed to better define the molecular bases of the different forms of CTCL, since this will facilitate the introduction of new, non-cytotoxic therapies.








