Abstract
The classification of lung cancer has evolved parallel to the knowledge of its biomolecular features and is implemented by the analysis of specific gene alterations, which have shown prognostic and predictive values. Consequently, the diagnosis of a specific ‘biomolecular subtype’ of lung cancer is accompanied by different therapeutic strategies.
Optimal target tissue sampling plays a key role in the diagnosis and treatment of lung cancer. Tissue samples can be obtained through various techniques involving different healthcare professionals. Therefore, a multidisciplinary approach is crucial to obtain a suitable diagnostic sample encompassing as much of the information as possible for optimal therapeutic management. In this paper, the authors share the expertise of all professionals involved in the diagnostic and therapeutic approaches of patients with lung cancer: pulmonologists, pathologists, oncologists, radiologists, surgeons, and molecular biologists. The different know-how contributions have been gathered in a single text to offer a comprehensive view on the management of the lung cancer tissue journey.
INTRODUCTION
In the era of target and immunotherapy, the multidisciplinary approach of lung cancer is now mandatory. Close collaboration between pulmonologists, radiologists, surgeons, oncologists, pathologists, and molecular biologists allows a high success rate in the management of lung cancer patients. In this review, different healthcare professionals suggest practical recommendations in the optimised tumour sampling to improve the diagnosis and treatment of lung cancer patients in daily clinical practice.
THE ONCOLOGIST’S VIEW ON PREDICTIVE BIOMARKERS
Targeting oncogenic drivers promoting and sustaining tumour cells has transformed the care of patients with lung cancer. Biomarker-targeted therapies in advanced non-small cell lung cancer (NSCLC) had a 62% cumulative success rate, far higher than the 11% observed in absence of a biomarker-targeted indication.1
However, the precision medicine approach requires tissue samples and/or liquid biopsy to be collected at baseline to evaluate predictive markers, and at tumour progression to identify the mechanisms of resistance to therapy. The need for a new tissue sample from metastatic disease, with the associated risk, cost, and discomfort, may be motivated by the lack of sample from the primary tumour, specific requirements of the analysis (most RNA and phospho-protein tests require fresh frozen samples), and in some instances the clonal evolution of the tumour.2,3 Sampling metastatic disease may only partially solve the problem of intratumour heterogeneity. Indeed, when many different clones are present in the patients’ metastases, the predominance of a selected neoplastic clone may change with time, appear in different locations, and in regard to the most aggressive clone, not be represented in the core biopsy. Alternative sources, including circulating free DNA, circulating tumour cells (CTC), and circulating micro vessels and exosomes, can be considered.4,5
Multiplexed testing enlarged to EGFR, ALK, ERBB2 (formerly HER2), BRAF, PIK3CA, MET, NRAS, ROS1, NTRK, RET, NRG, and other genes is fundamental to permit physicians to select therapies from first line of treatment and enrol patients in randomised trials.
Currently, testing a panel of predictive markers including EGFR and BRAF mutations, ALK and ROS1 rearrangements, and programmed death-ligand 1 (PD-L1) expression is mandatory in advanced NSCLC to ensure the most appropriate first-line therapy. A useful and adequate algorithm has been developed, suggesting the simultaneous determination of EGFR and BRAF mutations; ALK and ROS1 rearrangements; and PD-L1 expression in all advanced-stage non-squamous cell carcinomas, including never or former (<15 pack years) smokers with squamous cell carcinoma, and PD-L1 expression in advanced-stage squamous cell carcinomas (Figure 1). Following the approval of new promising biomarker-based drugs, a continuous updating of the algorithm will be required.6
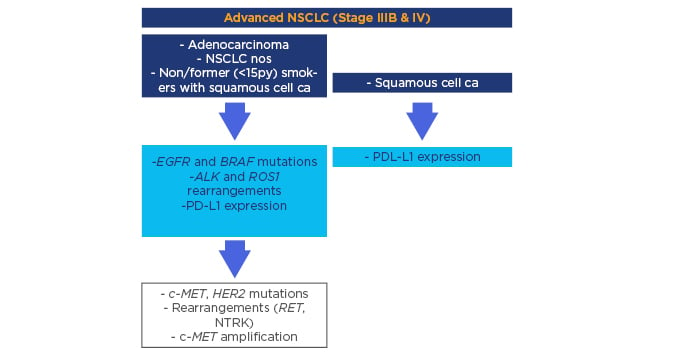
Figure 1: Clinico-pathologic algorithm describing the minimal requirements concerning predictive biomarkers in advanced non-small cell lung cancer starting with histology definition.
CA: carcinoma; EGFR: epidermal growth factor receptor; NOS: not otherwise specified; NSCLC: non-small cell lung cancer; PD-L1: programmed death-ligand 1.
THE RADIOLOGIST’S VIEW AND GUIDELINES FOR OPTIMISING BIOPTIC TARGETS AND IN RADIOLOGY
Indeterminate lung lesions are still a challenge for radiologists, oncologists, pulmonologists, thoracic surgeons, and pathologists. Lung tissue is obtained through minimally invasive techniques such as transbronchial biopsy/needle, trans-thoracic needle biopsy, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) within or without the oesophagus and can also be done with an echo bronchoscope to perform histologic classification, with or
without immunostains, and perhaps eventually with predictive biomarkers. Pre-procedural images allow technical planning including the selection of the most suitable imaging guidance, needle type, patient’s position based on preferred access routes, and scheduled number of samples. No validated indications are currently available on the needle size for each patient or lesion. While larger needles allow obtaining enough tissue for molecular tests, the needle size affects the risk of complications.7
Radiological diagnosis and staging of the lung lesions allow the appropriate selection of the patient referred to tissue sampling, the site of biopsy, and the bioptic technique (including the type of image guidance), provided that the images are carefully reviewed for the success of a biopsy, and that international, national, and institutional evidence-based guidelines are followed.8,9
In Italy, the Radiologic and Oncologic scientific societies (Italian Society of Radiology [SIRM] and Italian Association of Oncologic Medicine [AIOM], respectively)9,10 provide original guidelines on lung cancer by endorsing recommendations from the main international societies (e.g., Fleischner Society, International Association for the Study of Lung Cancer [IASLC], etc.), even if multidisciplinary guidelines are still lacking. The sequential imaging approach, as recommended by the AIOM guidelines, includes chest X-ray, compared with previous radiological studies when available; followed by a contrast-enhanced CT of thorax, abdomen, and brain; and finally by PET-CT. The classification based on the new IASLC 8th edition tumour node metastasis (TNM) staging system for NSCLC is generally recommended.11
In particular, integrated contrast enhanced CT and PET-CT can accurately depict the tumour site and size, the invasion of the surrounding structures, the nodal involvement, and the metastatic sites, combining them in a TNM stage.12 Based on this imaging and clinical staging (cTNM [including bronchoscopy and sometimes mediastinoscopy], thoracoscopy, EBUS-TBNA, EUS-FNA, and thoracentesis), each patient should be evaluated for curative surgical treatment by a multidisciplinary tumour board.
THE BRONCHOSCOPIST’S VIEW AND GUIDELINES FOR OPTIMISING BIOPTIC TARGETS
Several bronchoscopic biopsy techniques allow obtaining adequate cyto-histological samples for diagnosis and molecular characterisation of bronchial, pulmonary, and hilar-mediastinal-tumours. According to the location of the lesion, different approaches must be considered for sampling central endobronchial lesions, peripheral pulmonary lesions, and the pathological processes of the hilar-mediastinal area.
Central Endobronchial Tumours
Biopsy forceps are the most frequently used sampling instrument for endobronchial and submucosal lesions, with a sensitivity of 74–80%.13,14 The limitation of biopsy forceps is the complexity of sampling submucosal and peri-bronchial lesions, and the risk of missing diagnostic tissue from lesions with a superficially large necrotic component. In these cases, TBNA and forceps biopsy in combination may significantly improve the diagnostic yield of bronchoscopy; however, the cost of TBNA discourages its routine use. Cryobiopsy showed significantly higher diagnostic yield (95.2%) compared with forceps biopsy (82.2%) in central lesions suspected of malignancy;15 however, since it is an invasive procedure its use cannot be recommended routinely.
Peripheral Pulmonary Lesions
The diagnostic yield of bronchoscopic approach in these lesions is highly related to the size of the lesion, ranging from 5% to 64% for nodules <2 cm, to 30–75% for lesions >2 cm, and increasing to >80% for lesions with a diameter >4 cm.16 In recent years, new technologies have been introduced in the clinical practice for the transbronchial biopsy of peripheral pulmonary lesions as endobronchial ultrasound radial mini probes, and electromagnetic navigation systems.16 These new guidance technologies provide a higher diagnostic yield, especially for small lesions,17 but they have higher cost compared to fluoroscopy.
Pathological Processes of the Hilar-Mediastinal Area
The only device available to obtain diagnostic samples from lesions of the hilar-mediastinal area is the transbronchial needle. TBNA demonstrated its efficacy both in the staging of lung cancer and in the diagnosis of mediastinal pathology with a diagnostic yield of 78%.18 The diffusion of EBUS-TBNA has allowed a great improvement in the diagnosis of hilar/mediastinal lesions, with a diagnostic sensitivity of >90%.19 Furthermore, the combination of bronchial EUS-FNA with EBUS-TBNA allows a complete evaluation of the extent of the tumour by complementary access to different stations (nodes 8, 9, and 5).20
The Role of Rapid On-Site Cytological Evaluation in Optimising of Specimens
Rapid on-site cytological evaluation (ROSE) of cytologic smears is recommended as an assessment of adequacy of the specimens, regardless of sampling procedure. ROSE allows immediate quality assessment of the specimens collected during bronchoscopic procedures, especially transbronchial needle aspiration techniques. Based on this information, the operator determines whether additional material needs to be collected and assigned to further analyses required for target therapy setup. Although there is no evidence of increased sensitivity in TBNA or EBUS-TBNA by using ROSE, the immediate cytologic assessment reduced the number of needle passes and complication rates, and improves the adequacy of samples for molecular evaluation.21
THE THORACIC SURGEON’S VIEW AND GUIDELINES FOR OPTIMISING BIOPTIC TARGETS
Mediastinoscopy
Mediastinoscopy and video-assisted mediastinoscopy (VAM) are considered the reference standard for mediastinal staging of lung cancer, providing access to ipsilateral and contralateral superior mediastinal lymph nodes (stations 2L/R, 4L/R, and 7), with a sensitivity of 78% and 89%, respectively.22 Surgical staging is indicated if negative results on EBUS/EUS are obtained and VAM is preferred to traditional mediastinoscopy.23 Mediastinoscopy is very useful when a larger sample of tissue is needed for conclusive diagnosis and molecular profiling. Furthermore, VAM is advisable to provide histological information for small lymph nodes (<5 mm), or for restaging after neoadjuvant treatments. 23
Video-Assisted Mediastinal Lymphadenectomy and Transcervical Extended Mediastinal Lymphadenectomy
Video-assisted mediastinal lymphadenectomy and transcervical extended mediastinal lymphadenectomy are invasive techniques that enable systematic lymph node dissection instead of sampling to improve the accuracy of transcervical surgical mediastinal staging.24,25 These are not yet considered standard procedures and should be performed in very experienced centres because of their potentially higher morbidity (1.0–5.0%) and mortality (0.3–6.6%).
Video-Assisted Thoracoscopic Surgery
Video-assisted thoracoscopic surgery allows for the obtaining of large tissue samples of every mediastinal node station, and an accurate staging by exploring pleural space and performing pleural and lung biopsies. With a sensitivity of 96–100%, video-assisted thoracoscopic surgery can be considered a useful and safe alternative to traditional mediastinoscopy when it is contraindicated, not suitable, or not available, such as in the case of enlarged PET-positive para-aortic (station 6) and sub-aortic (station 5) nodes that are easily reached.26
THE PATHOLOGIST’S VIEW AND GUIDELINES FOR OPTIMISING TUMOUR TISSUE
The role of the pathologist is radically changed in the era of targetable oncogenes. The main barrier to attain a complete panel of biomarkers is accountable to the small amount of tissue available in small biopsy and cytology samples. Histology still has a role in selecting chemotherapy in PD-L1 negative and non-oncogenic driven NSCLC, and in guiding predictive molecular determinations. The increasing pressure for predictive biomarkers following first-line treatment will require larger tumour tissue samples, and a correct handling of small biopsy and cytology to minimise failure rates.27 Pathologists are called to improve and maximise the use of tumour tissue available by using several intra-laboratory approaches, such as the following:
Limiting Diagnostic Immunohistochemical Markers for Non-Small Cell Lung Cancer Subtyping
According to the World Health Organization (WHO) lung tumours classification,28 morphology on haematoxylin-eosin (H&E) stain is sufficient to define histology in the vast majority of cases. Even in cases of a poorly differentiated NSCLC, it is important to restrict the immunostains at a minimum (TTF-1 and p40).29
Cell Block Preparation
Since various molecular biomarkers may be detected in lung cytologic specimens, the preparation of cell block (CB) is currently recommended. CB retains tissue architecture and provides multiple additional sections of various thickness for ancillary in situ or extractive analyses; moreover, CB may be archived. Different CB preparations are available with various yields. Plasma thrombin, direct clotting, and gel-based preparations are the most common, while collodion bags apparently give a higher cellular yield.30 Formalin and alcohol are the most used fixatives.31 When CB is prepared during rapid on-site evaluation, the first smeared slides (air-dried for Diff-Quick and alcohol fixed for Papanicolaou stains) should be used to state the adequacy of the presence and the amount of tumour cells, while all the remaining material should be dedicated to CB preparation. CB preparation may be obtained even from previous smears from FNA cytology, decolourising and removing the tumour cells, and then proceeding to CB preparation. This technique, called ‘cytoscrape’ may be performed even from archival material of NSCLC by gently scraping tumour cells off the slides to perform cellular clots.32 CB provides a precious source of tumour cells; therefore, the entire volume from pleural or pericardial effusions should be submitted to centrifugation and have the pellet recovered, regardless of the number of molecular tests to be performed and the abundance of other available specimens.
Tumour Enrichment by Microdissection
To achieve high-quality molecular testing, the pathologist should mark the most suitable tumour area on the slide so that the optimal tumour content is extracted from cytology and paraffin-embedded material. Necrosis, bloody areas, mucous plugs, and inflammatory infiltrate should be eliminated from the selected material for fluorescent in situ hybridisation (FISH) analysis or extractive methodologies. Tumour cell microdissection attains a very high tumour/non-tumour cell ratio allowing the enrichment of tumour material. Moreover, the identification and selective isolation of tumour cells can be obtained from H&E stained slides examined under a light microscope. Laser-capture microdissection is an alternative method to perform tumour enrichment, albeit expensive and time-consuming. The ratio between tumour cells and non-tumour cells is of critical importance and, if possible, a minimum of 20–30% of tumour cells should be present in the material tested for genetic alterations to minimise false-negative results. Direct sequencing required at least 20–30% enrichment, and even a modern next-generation sequencing (NGS) approach may require at least 10% enrichment. Technologies based on real time PCR are needed for specimens with enrichment of 1–10%. Digital PCR may even allow analysis of specimens with tumour cell enrichment <1%, but its application is currently discouraged in tissue samples.
High Throughput Technologies
The introduction of NGS technologies in routine practice might permit a comprehensive characterisation of all current and next targetable genomic alterations (mutations and gene fusions) from relatively limited sources of tumour tissue,33,34 therefore preventing the necessity to perform immunohistochemistry (IHC) and/or FISH. EGFR and ALK co-alterations were detected in up to 8% of cases using high-sensitivity peptide nucleic acid probe-based real-time PCR, ultra-deep NGS, and mutant-enriched NGS, with clinical benefits.35 Due to the ability of NGS techniques to detect hot spot mutations, copy number variations, and gene fusions at the same time, NGS platforms will likely replace the majority of other technological assets in molecular pathology. Several papers recently highlighted the efficiency and cost-effectiveness of NGS panels in simultaneously testing all predictive biomarkers,36 possibly overriding the need for NSCLC subtyping.37 Other high throughput methods are now available for the detection of multiple gene fusion transcripts, including the NanoString technology.38
Standardised Operative Procedures
After a minimised transfer time of tumour tissue from the operating rooms to the pathology laboratory, the samples should be immediately fixed in buffered formalin for 6–48 hours. To maximise tissue availability, it is important to perform minimally invasive sectioning during the preliminary diagnosis. Experienced technicians appropriately cut the paraffin block to expose complete surface of the bioptic fragments on the initial slides, without losing material for diagnostic ancillary techniques and molecular testing (microtomes equipped with ‘waterfall’ slides and 2–3 µm thick slides work perfectly for IHC). Since re-cuts should be kept at a minimum, another option is to cut multiple (approximately 20) unstained sections and keep them stored until a preliminary diagnosis has been made and ancillary testing is requested. This avoids tissue waste and shortens turnaround times (5–10 days), saving tissue and reducing costs.39
Multiple Blocks from Multiple Biopsies/Single Biopsy
Separation of multiple fragments into multiple paraffin-embedded blocks may maximise the probability of successful molecular testing, consuming less tumour surface in a unique slide and saving tissue (separate blocks) for further molecular analysis, or material to submit for clinical trials.33 Since PD-L1 expression is characterised by intratumoural heterogeneity, this determination should be tested along with the initial H&E-stained slide to cover the entire tumour surface, then the bioptic fragments should be separated into different blocks.40 The authors strongly discourage alternative approaches using separate biopsies for routine practice and pathologist-uncontrolled molecular determinations.41
Management of Large Resected Samples
The optimisation of tissue sampling and handling can also be important for surgically resected samples from large tumours with heterogeneous features. In these cases, care should be taken in the fixation step, due to the slow penetration of formalin in tissue (5 mm per hour), as well as in tumour sampling, to accurately avoid necrotic areas. Moreover, considering the issue of tumour heterogeneity, the collection of different tumour areas in single paraffin blocks is advisable to maximise the detection of clinically important biomarkers in large tumours.
Reflex Testing
In eligible NSCLC patients, who do not receive the results of predictive biomarkers at their initial oncology consultation, the correct treatment is significantly delayed and frequently a second biopsy is performed to guarantee appropriate molecular testing after the initial consultation. About 19% of patients started chemotherapy before biomarker results became available. This can be avoided by incorporating reflex biomarker testing into diagnostic algorithms for NSCLC at the pathology level, and by further educating specialists to provide sufficient diagnostic cancer specimens for molecular testing.39
Tissue Decalcification
Decalcification procedures may complicate the results of biomarker determinations.33 Decalcification irreversibly damages nucleic acids and prevents PCR-based molecular techniques as well as FISH. Therefore, bone biopsy should be avoided when the tumour target can be reached in a different site. It is good practice to warn the laboratory and address the bone biopsy directly to the pathologist involved in preparing the tumour tissue for molecular biomarker determination.42 To circumvent tissue decalcification, pathologists should separate calcified from fleshy material, processing the latter separately without decalcification. There are new
available reagents for decalcification based on ammonia or ethy lenediaminetetraacetic acid that better preserve nucleic acids.
Different Determinations from a Single Sample
IHC and FISH analyses should be performed as a first step, exploiting the possibility to perform EGFR mutation analysis on the same sample previously submitted to ALK and/or PD-L1 IHC/FISH procedures, by scraping off tumour cells from stained slides.39,43 Due to alcohol fixation, smeared cytology usually provides an excellent preservation of nucleic acids and smear areas highly enriched in tumour cells can be scraped for successful DNA extraction and sequencing.
The Minimal Amount of Tissue to Detect all Biomarkers in Routine Practice
The minimal amount of tumour tissue for adequate biomarkers testing depends on the type of genetic alteration to investigate, the sensitivity of the method, the type of tissue, and the laboratory expertise. EGFR mutations may be appropriately detected using a tumour/non-tumour tissue ratio >30% with ≥100 tumour cells. Detection of ALK rearrangement using FISH testing requires ≥50 tumour cells on biopsy or cytology, and ≥20 tumour cells are needed when ALK rearrangement is indirectly detected by means of IHC.33,39,43 PD-L1 expression requires a minimum of 100 tumour cells.
The Molecular Biologist’s View on ReBiopsy, Liquid Biopsy and Next-Generation Sequencing
Oncogene-driven and ‘druggable’ tumours undergoing pharmacologic resistance, for which an alternative therapy is available, require tissue rebiopsy, and all methods and guidelines described above play a major role.
Histologic change is a possible mechanism of drug resistance not only in various oncogene-driven settings (e.g., EGFR mutations, ALK rearrangement) and during immunotherapy, but also in wild-type NSCLC.44
Circulating tumour DNA (ctDNA) and CTC are important tumour-derived materials obtained from the bloodstream, which are a promising source of fresh material for detection of molecular biomarkers, for both diagnostic and monitoring purposes when tumour tissue is lacking. CTC, ctDNA, and other cancer-related material in the bloodstream are commonly known as ‘liquid biopsy’. The technique is less invasive, repeatable, and rapid, possibly bypassing tumour heterogeneity.45-47
Studies comparing the sensitivity and specificity of ctDNA analysis to tissue biopsy in NSCLC confirmed the clinical value of this alternative approach.48
Recently, a valuable use of liquid biopsy has been proposed in the selection of metastatic EGFR-T790M-positive NSCLC patients, who have progressed on or after first-line therapy with EGFR tyrosine kinase inhibitors, eligible for treatment with osimertinib. The National Comprehensive Cancer Network (NCCN) guidelines reported that plasma genotyping may be considered at progression instead of at biopsy to define the eligibility for treatment with osimertinib; however, if plasma testing is negative, then tissue biopsy is recommended.49
Availability of a potential blood-based test for ctDNA means that alternative biomaterials sources could be useful to select metastatic NSCLC patients sensitive to specific target therapy.
In the future, NGS-based liquid biopsy might represent an emerging approach in the treatment decision-making of metastatic lung cancer patients because it could ensure a comprehensive genomic profiling using biomaterial obtained through minimally invasive procedures (Figure 2). Moreover, NGS-based liquid biopsy could have a great advantage in overcoming spatial heterogeneity linked to tissue biopsy.50
At present, diagnosis and subtyping of lung cancer require a histology-based analysis; therefore, the co-ordinated use of tumour tissue from simultaneous sampling of conventional biopsy/cytology and ‘liquid biopsy’ is the preferred approach.
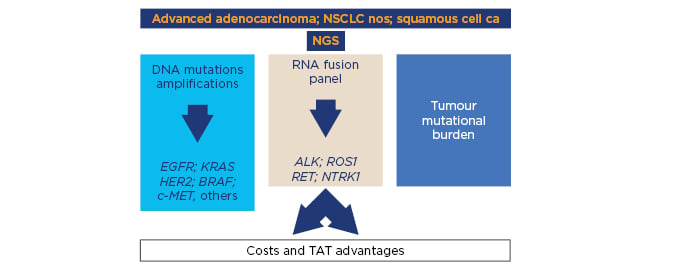
Figure 2: Next-generation sequencing including DNA and RNA analysis to simultaneously detect tumour cell mutations and rearrangements is a promising approach to perform multiple determinations using a limited amount of tumour tissue in a relatively short turnaround time and several cases.
CA: carcinoma; NGS: next-generation sequencing; NOS: not otherwise specified; NSCLC: non-small cell lung cancer; TAT: turnaround time.
CONCLUSION
Several molecular gene alterations have been unevenly identified in lung cancer, particularly in NSCLC with adenocarcinoma histology. The discovery of oncogenic drivers has led to the development of drugs tailored to the tumour molecular profile permitting a significantly higher clinical response and survival among biomarker-selected patients. Molecular-driven information allows innovative clinical trials and negates histology-dependent therapies. In this scenario, tissue biopsy still represents the gold standard for molecular analysis, but noninvasive or minimally invasive liquid biopsy methods are entering clinical practice, providing more tumour tissue for optimising molecular determinations and monitoring disease progression.
As exemplified in this comprehensive review, a close multidisciplinary collaboration among oncologists, pathologists, molecular biologists, radiologists, bronchoscopists, and thoracic surgeons is the only way to decide the best site to sample in obtaining more tumour tissue, limiting the procedural invasiveness, and furnishing the best neoplastic material for molecular analyses.








