Abstract
Pleural malignancies constitute either primary pleural malignancies, such as malignant pleural mesothelioma (MPM), or secondary pleural tumours, either from pleural metastasis or direct extension of adjacent tumours. Mesothelioma is a rare aggressive tumour of the pleural surfaces associated with prior asbestos exposure. Mesothelioma is also a challenging disease from a diagnostic staging, and treatment perspective and is rarely cured despite multimodal treatment. With incidence continuing to rise, this disease represents a serious global problem that needs urgent attention.
This review provides an in-depth review of MPM. Recent advances in diagnostic approaches, such as imaging techniques and the role of immunohistochemistry and biomarkers, are discussed. Treatment modalities, including chemotherapy, radiotherapy, and surgery as part of a multimodal approach, are reviewed, as well as the management of malignant pleural effusions.
INTRODUCTION
Malignant pleural mesothelioma (MPM) is a rare aggressive tumour derived from the mesothelium that invades the pleura. It is a challenging disease from a diagnostic, staging, and treatment perspective and is rarely cured despite multimodal treatment. During the past 20 years, important advances in the diagnosis and management of MPM have occurred; however, much is still left to be understood about this devastating disease. In this paper we provide an in-depth review of MPM with a focus on recent advances in imaging and biomarkers.
EPIDEMIOLOGY
Prior asbestos exposure is a critical risk factor for MPM, with 80% of cases caused by occupational exposure. Environmental exposure to asbestos (erionite fibres) naturally existing in the soil of areas such as Turkey, Corsica, and Cyprus, and neighbourhood exposures in people living close to asbestos factories, have also been described as risk factors for MPM. Paraoccupational exposure of household members to asbestos from the clothes of asbestos workers, as well as exposure to ceramic refractory fibres, ionising radiotherapy, and simian vacuolating virus 40, have also been linked to MPM.1
With an increase in industrialisation over the last two decades, the incidence of MPM is predicted to peak during 2020–2025.1 The World Health Organization (WHO) has estimated that 92,250 deaths occur annually from asbestos-related disease. MPM portends extremely poor outcomes, with a median survival of 9–12 months. It has a 4:1 male predominance, and women have more favourable outcomes. MPM is subdivided into four histological subtypes: epithelioid, sarcomatoid, biphasic, and desmoplastic. The sarcomatoid type of MPM is associated with the poorest prognosis, with a median survival of 4 months.2
DIAGNOSIS
The ability to differentiate between MPM, benign pleural tumours, and pleural metastases requires multimodal evaluation. To date, there is no single diagnostic test with adequate sensitivity and specificity for early diagnosis in asymptomatic subjects. Imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), play a key role in the assessment of patients with suspected MPM. Depending on the presence of pleural effusions or thickening, the extent and laterality, and the presence of calcified pleural lesions, the contribution of imaging can differ significantly, from providing diagnostic or staging information to differentiating benign from malignant pleural thickening. Radiological interpretation is more challenging in early disease with minimal or absent pleural thickening, and staging can be difficult due to the heterogeneous growth patterns of MPM.
Ultrasound
Contrast-enhanced thoracic ultrasound can quantify pleural effusions or thickening, identify nodules on the pleura or hemidiaphragm, and evaluate their degree of vascularisation. Pleural-based lesions, pleural thickening >1 cm, nodular pleural thickening, and diaphragmatic nodules have >95% specificity for malignancy. The low sensitivity of 42% for differentiating malignant from benign disease, and wide interoperator variability, however, makes further evaluation of non-specific findings imperative, particularly with negative cytology from thoracentesis.3
Computed Tomography
The features on CT scans that distinguish MPM from metastatic pleural disease include circumferential pleural thickening (pleural rind), mediastinal pleural involvement, and a pleural thickness >1 cm. While contrast-enhanced CT is the recommended initial approach for evaluation, it has a modest sensitivity (58%) and specificity (80%) for diagnosing pleural malignancy. Around 32% of patients with pleural effusion have malignancy based on histology, despite negative findings on CT.4 CT cannot reliably differentiate MPM from pleural metastases or between MPM subtypes. CT also has a limited role in the staging of MPM, as it is suboptimal for detecting mediastinal lymph node metastases and underestimates early chest wall invasion and diaphragmatic and peritoneal involvement. This is because mesothelioma has similar tissue attenuation to nearby structures, including the chest wall musculature, diaphragm, and pericardium.5
Positron Emission Tomography and Positron Emission Tomography-Computed Tomography
PET cannot reliably assess the extent of local tumour invasion and cannot differentiate MPM from pleural metastasis or between histological subtypes. Moreover, PET is associated with high false-negative rates in early disease6 and high false-positive results in tuberculous pleuritis, parapneumonic effusions,7 and prior pleurodesis.8 The use of integrated PET-CT combines metabolic with anatomical information, providing improved diagnostic and staging accuracy. It is superior to MRI, PET, or CT alone for diagnosing MPM and outperforms CT and MRI in detecting intra-thoracic and extra-thoracic lymphadenopathy and extra-thoracic metastatic disease.9 Despite its high sensitivity (88%) and specificity (93%), PET-CT does not perform well in detecting lymph node micro-metastases, specifically in N2 disease.9 Surgical staging by endobronchial ultrasound (EBUS), mediastinoscopy, or oesophageal ultrasound fine needle aspiration, is recommended.10
Magnetic Resonance Imaging
MRI plays a significant role in pre-operative evaluation of MPM cases that show suspected local invasion on CT scans. It can detect chest wall or extrapleural invasion, such as mediastinal or diaphragmatic extension, more reliably than CT. MRI also has a sensitivity of 85% and specificity of 100% for detecting T3 disease or greater, but sensitivity drops to 23% for T2 disease or less.11
Pleural Biopsy
Histopathological confirmation is the gold standard for diagnosis of MPM. Therefore, obtaining adequate samples from pleural biopsies, both in quantity and quality, is essential. Current guidelines recommend at least five biopsies >10 mm of normal and visibly abnormal parietal and visceral pleura, and sub-pleural tissue.1 CT-guided biopsy is preferred over ‘blind’ or closed needle biopsy, as the latter leads to inaccurate and smaller biopsy samples due to the lack of proper visualisation of the sampling point. Newer techniques, such as CT-guided cutting needle pleural biopsy, have high sensitivity (91%), specificity (100%), and accuracy (91%) for diagnosing malignant lesions.12 When compared to medical thoracoscopy, CT-guided Abrams’ needle pleural biopsy had equal diagnostic sensitivity (88%). The low complication rates make it a safe procedure in patients with suspected MPM.13
Open biopsies via pleuroscopy (medical thoracoscopy) or video-assisted thoracoscopic surgery (VATS) allow for multiple, large, and deep biopsies and direct visualisation of visceral or diaphragmatic pleural involvement. They therefore have higher diagnostic yields and sensitivities (95%), allowing for more accurate staging.14 Pleuroscopy is less invasive and can be performed under conscious sedation, while VATS requires general anaesthesia and single-lung ventilation. The more extensive approach, however, allows for combined diagnosis and treatment in one procedure. VATS with mediastinoscopy is recommended when mediastinal nodal involvement is suspected.14
Endobronchial Ultrasound
For nodal staging, EBUS provides an accurate diagnosis with minimal complication rates, and allows access to the hilar lymph nodes that are inaccessible with mediastinoscopy.10,15 Some studies report the superior performance of EBUS over mediastinoscopy, with a sensitivity and negative predictive value of 59% and 57%, respectively, for EBUS, compared to 28% and 49%, respectively, with mediastinoscopy.15
Immunohistochemistry
Immunohistochemistry (IHC) is critical for differentiating MPM from metastatic carcinomas. It is most helpful in differentiating epithelioid MPM from pleural metastasis of epithelial malignancies, such as primary adenocarcinoma of the lung. Current guidelines recommend the use of monoclonal antibody panels16 with at least two positive mesothelial markers and two negative carcinoma markers for the diagnosis of MPM.1,16,17 The use of thyroid transcription factor 1 and napsin A can help differentiate epithelioid MPM from lung adenocarcinoma.16 Other organ-specific markers can be used to exclude metastatic disease from other primary sites.1,16,18 Commonly used IHC markers are listed in Table 1.
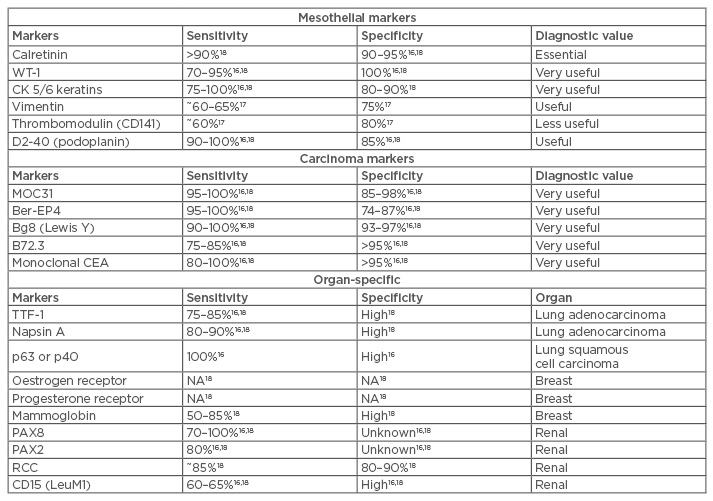
Table 1: Immunohistochemical markers used in the differential diagnosis between epithelioid malignant pleural mesothelioma, lung carcinoma, and other carcinomas.
IHC has a limited role in sarcomatoid MPM. Sarcomatoid tumours express pan-cytokeratins, vimentin, and markers of smooth muscle differentiation, such as smooth muscle actin, but test negative for most mesothelial markers, with the exception of D2-40 and calretinin. Because of this, the diagnosis of sarcomatoid MPM requires at least two pan-cytokeratins, two non-mesothelial markers, and supporting clinical or imaging data.1,16
Non-Immunohistochemistry Biomarkers
There has been increasing interest in the role of biomarkers for earlier diagnosis of MPM. Their clinical application, however, is characterised by low sensitivity, specificity, and reproducibility, with variable results reported by published studies.
Serum mesothelin
The most widely used biomarker is serum mesothelin. Elevated levels of serum mesothelin are common in patients with MPM compared to patients with pleural metastases or asbestos-related benign pleural disease.19 Conflicting data exist regarding the role of soluble mesothelin-related peptides (SMRP) as a diagnostic biomarker. While SMRP have high specificity (95%), they have sub-optimal sensitivity (32%), being negative in both sarcomatoid and in half of epithelioid subtypes, especially in the early stages.20 Although pleural fluid SMRP biomarker performs better than its serum counterpart, its utility in pleural fluid samples limits its role as a screening tool for early disease, where pleural effusions are uncommon. Its greatest role is in monitoring treatment response, as SMRP levels correlate with tumour size and progression.21
Osteopontin
Osteopontin (OP) is a glycoprotein that is overexpressed in several malignancies, including MPM. Its role as a useful biomarker for diagnosis and prognosis of MPM has been studied thoroughly. Pass et al.22 found that OP levels in tumour tissue, but not serum, were significantly elevated in MPM when compared to healthy controls with and without asbestos exposure. Moreover, both serum23 and plasma22 OP levels correlated with survival. Studies on the diagnostic power of OP, however, have produced conflicting results. This is because OP in serum is unstable, due to thrombin cleavage during the coagulation process, leading to unreliable results.23 Elevated OP levels have also been associated with other malignancies, causing a low diagnostic specificity. Plasma OP, on the other hand, may have better diagnostic performance.24
Hyaluronan
Hyaluronan (HA), a polysaccharide expressed in high levels in the serum and pleural fluid of patients with MPM, is another established biomarker. Pleural fluid levels of >100,000 ng/mL have been recommended as a diagnostic indicator for MPM,25 and elevated intracellular HA levels have also been associated with MPM.26 Perhaps its greatest contribution, however, lies in its ability to differentiate between MPM and metastatic adenocarcinoma, since mesothelioma cells express high levels of intracellular HA, a feature not found in metastatic adenocarcinoma.27 Serum HA levels are higher in patients with later or progressive stages compared to responders, suggesting that HA is a marker of progressive disease.28
MicroRNA
MicroRNA (miRNA) are short, non-protein coding single-stranded RNA involved in the regulation of gene expression and can contribute to either oncogenesis or tumour suppression. They are stable tissue-specific molecules that can differentiate mesothelioma from pleural metastases. Several studies have explored different miRNA expression profiles in MPM tissues, serum, and pleural fluid, using microarray profiling and quantitative real-time polymerase chain reaction. These studies have helped determine a subset of miRNA that are differentially expressed between MPM and healthy tissue. Specific miRNA for each histopathological subtype have also been identified. For example, miR-126 has been consistently shown to be downregulated in MPM tissue compared to normal pleura.29 Despite its high sensitivity, miR-126 lacks tumour specificity since it is also expressed in other malignancies. miR-126 downregulation, in combination with established biomarkers such as mesothelin, could possibly be used for early detection of MPM. Plasma miRNA, such as miR-625-3p,30 and two distinct serum miRNA31 have also been validated as diagnostic markers for MPM.
SOMAscan™
Protein analysis with a 13-protein biomarker detection assay (SOMAscan™) has gained interest for diagnosing MPM, with high sensitivity (94%) and specificity (91%). Although larger studies are needed to validate its diagnostic performance, this novel technique could also identify potential targets for treatment.32
STAGING
Staging of MPM is based on the recommendation by the International Mesothelioma Interest Group, where TNM classification of the primary tumour, lymph node involvement, and distant metastasis is followed. The 8th edition of TNM Staging for MPM is detailed in Table 2.33
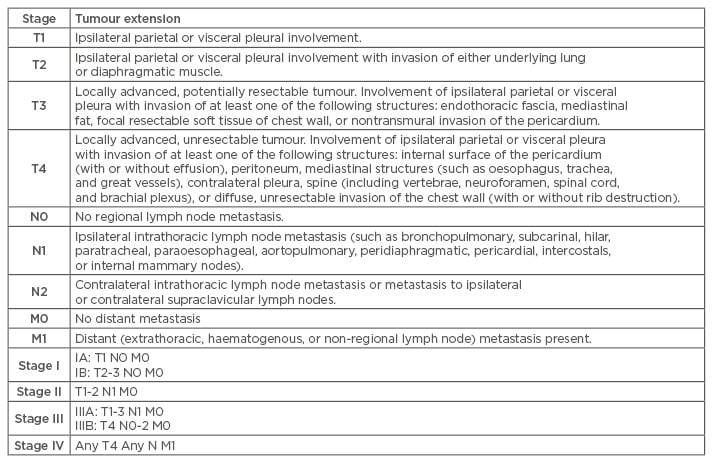
Table 2: 8th edition of TNM classification of malignant pleural mesothelioma from the International Mesothelioma Interest Group.33
TREATMENT
Treatment remains the most challenging aspect of MPM because it responds poorly to chemotherapy and radiation therapy (RT), and surgery is rarely curative. A multidisciplinary approach to define best treatment strategy is preferred. Treatment is dictated by the extent of tumour invasion and pre-operative TNM staging. Unfortunately, staging is often corrected intra-operatively after direct visualisation of tumour extension.34
Chemotherapy and Immunotherapy
Irrespective of surgical resection, chemotherapy improves survival in MPM.35 Combination therapy with cisplatin or carboplatin with pemetrexed or raltitrexed is first-line and confers a survival benefit when compared to cisplatin alone.36 Several trials are currently studying immunomodulators, including the vascular endothelial growth factor inhibitor, bevacizumab,37 and tyrosine kinase inhibitor, sunitinib.38 Pembrolizumab, an anti-programmed cell death receptor 1 antibody, shows promising results in patients with programmed cell death ligand 1 positive MPM.39 Randomised studies, however, are needed to confirm these results. Because mesothelin is highly expressed by mesothelioma cells, it makes a suitable target for immunomodulators. Several anti-mesothelin therapies, such as amatuximab, a chimeric monoclonal antibody,40 and recombinant immunotoxins,41 have shown improved response rates and overall survival in smaller studies. These drugs are currently being studied in larger, randomised clinical trials.
Radiation Therapy
While RT has not demonstrated a survival benefit in MPM, it plays an important role in palliation and symptom management. RT to large areas of the body, such as the whole hemithorax, carries significant risks of organ toxicity, especially to the lung, liver, heart, bone marrow, and oesophagus.42 Although improved techniques and use of intensity-modulated RT (IMRT) have diminished toxicity while providing adequate radiation, RT-induced pneumonitis remains a significant problem.
For palliation, a short course of RT is often used to relieve the chest pain from chest wall invasion.43 Prophylactic RT directed to pleural intervention sites is no longer recommended prior to thoracoscopy or thoracotomy, due to the lack of evidence showing a reduction in tumour seeding through these scars.44 RT can be given as part of adjuvant treatment after chemotherapy and surgery to control residual microscopic disease. The large surface of the pleural space and tumour growth in the diaphragmatic creases and the lobar fissures, however, require high doses to achieve local control, which exponentially increases toxicity risk.45 Some trials, however, report acceptable toxicity with ipsilateral RT after lung preservation surgery.46 Ongoing studies, including the Prophylactic Irradiation of Tracts (PIT) trial, aim to identify optimal timing for post-surgical IMRT.47
Surgery
Surgery via extrapleural pneumonectomy (EPP) or extended pleurectomy/decortication (P/D) is used for staging and curative intent. EPP involves complete removal of the visceral and parietal pleura and the ipsilateral lung, resection of the ipsilateral pericardium and diaphragm, and dissection of mediastinal lymph nodes. P/D involves removal of the pleura and release of the lung and chest wall from constriction caused by the tumour, without pneumonectomy.48 With either technique, the main objective is complete macroscopic and microscopic resection of all malignant growth. Unfortunately, obtaining negative resection margins is extremely difficult to achieve intra-operatively, due to the proximity to adjacent structures.
EPP is a more extensive surgery, reserved for patients who are candidates for multimodal therapy with neoadjuvant chemotherapy and post-surgical RT. While mortality is low at 5%, complications, including cardiac and respiratory failure, empyema, and bleeding, are common.49 P/D involves fewer complications at the expense of higher recurrence rates. It is used for patients with diffuse parietal involvement but small local visceral invasion. P/D can be performed using VATS, further reducing the morbidities associated with thoracotomy and allowing for simultaneous pleurodesis intra-operatively.50 Several studies that aimed to determine the best surgical technique have failed. A retrospective review of 663 patients showed slightly increased survival with EPP. These findings, however, were thought to be largely due to selection bias and patient factors.51 The MARS trial compared EPP with no EPP after neoadjuvant chemotherapy. The feasibility study could only enrol 45 patients and, even in this small cohort, survival benefit, albeit small, was in favour of no EPP.52 A follow-up study, MARS-2, that aims to compare P/D versus no P/D is currently enrolling patients.53
Several studies have examined the feasibility and safety of sequential multimodal therapy using neoadjuvant chemotherapy followed by surgery and RT. While some studies report improved 90-day mortality rates, the biggest limitation has been high complication rates and feasibility, with less than half of patients successfully enrolled.52 Currently, the most widely accepted strategy in patients with acceptable functional status and limited disease is multimodal therapy, in specialised centres only.1
Pleurodesis
Malignant pleural effusions (MPE) occur frequently with MPM. The diagnosis of MPE is an ominous sign of widespread metastasis and portends a grave prognosis, with median survival of 4 months. MPE can decrease quality of life due to dyspnoea, cough, and chest pain.
Treatment is tailored towards symptomatic relief with pleurodesis to prevent recurrent effusions. Chest tube pleurodesis requires drainage of the pleural effusion via chest tube insertion, followed by installation of sclerosing agents into the chest tube. Sterile talc is the most effective sclerosing agent and is therefore preferred over tetracycline, bleomycin, or doxycycline. Thoracoscopic pleurodesis can be performed by VATS or pleuroscopy, where mechanical pleural abrasion precedes instillation of sclerosants into the pleural space. VATS pleurodesis is the preferred method because it allows for better distribution of talc and release of adhesions, causing complete lung expansion and improved apposition of pleural surfaces.1 Pleurodesis should be considered in patients with life expectancies of >3 months, whose symptoms are relieved with therapeutic thoracentesis. Pleurodesis may be difficult to achieve in patients with trapped lungs or multiple loculations, as it requires the lung to expand against the chest wall with apposition of visceral and parietal pleural membranes. Pleurodesis is most effective when performed early. Small bore chest tubes (<16 Fr) are associated with reduced pain compared to large bore tubes and are equally effective in achieving pleurodesis.54 Pleurodesis is difficult to achieve in MPM, due to lung entrapment by the tumour, the presence of the tumour in the pleural cavity, and the resistance of malignant mesothelial cells to talc pleurodesis.55
Placement of a tunnelled pleural catheter (TPC) should be considered for symptom relief, particularly in patients with unexpandable lung or failed pleurodesis. It is as effective at relieving symptoms as talc slurry via chest tubes, and can sometimes lead to spontaneous pleurodesis.56,57 Placement of the TPC can be carried out at the bedside or as an outpatient procedure. Complications include infection, displacement, catheter tract metastases, and tube blockage.57 An ongoing trial, IPC PLUS, is looking at the efficacy of TPC placement versus TPC and talc pleurodesis, and may provide us with improved strategies for management of MPE.58
FUTURE DIRECTIONS
Although progress is being made for diagnosing MPM, treatment is not advancing so quickly. Identifying biomarkers for earlier disease detection, perfecting protocols for multimodal treatment, and developing novel therapeutic approaches are of utmost importance. Promising advancements in diagnostic tools, such as novel biomarkers in pleural effusions and serum, as well as IHC and signature miRNA, will help with earlier detection and accurate tumour profiling. Advancements in therapies, including immunotherapy, intrapleural gene therapy via adenovirus,59 exosome-delivered miRNA, and other drug delivery systems,60 as well as TPC with a drug-eluting coating to deliver intra-pleural sclerosing agents over time,56 offer hope for a better diagnosis and treatment of this disease.








