Interviewees: George W. Sledge Jr,1 Antonio Llombart-Cussac2
1. Division of Oncology, Stanford University School of Medicine, Stanford, California, USA
2. Medical Oncology Department, Hospital Arnau de Vilanova, Valencia, Spain
Disclosure: Prof Sledge has received clinical trial support, research grants, and travel/accommodation/expenses from Eli Lilly and Company; research grants from Pfizer; is on the board of directors for Tessa Therapeutics; and has been a consultant for Syndax, Symphogen, and Verseau Therapeutics. Dr Llombart-Cussac has received clinical trial support, research grants, speaker’s honoraria, and travel/accommodation/expenses from Eli Lilly and Company, Pfizer, and Roche-Genentech; research grants from EISAI, MSD, Novartis, Genomic Health, and AGENDIA; and speakers honoraria from EISAI, Pierre-Fabre, Genomic Health, and AstraZeneca; and is co-founder and stockholder with MedSIR.
Acknowledgements: Medical writing assistance was provided by Dr Brigitte Scott, MarYas Editorial Services, Cowlinge, UK
Support: This article was funded by an educational grant from Eli Lilly and Company.
Disclaimer:The opinions expressed in this article belong solely to the named interviewees.
Citation: EMJ Oncol. 2020;8[Suppl 2]:10-20.
INTRODUCTION
The introduction of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors in the treatment of advanced breast cancer (ABC; inoperable locally advanced or metastatic breast cancer) has changed the treatment landscape of this disease. CDK4/6 inhibitors in combination with endocrine therapy (ET) are now considered a standard-of-care treatment for patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) ABC. Understanding the efficacy of these inhibitors in clinical trial populations and in specific subgroups thereof is providing new possibilities for the management of all ABC patients in the clinic, including previously difficult-to-treat cases. Awareness of the toxicity profile of these drugs is also enabling physicians to help oncologists select CDK4/6 inhibitors and for patients to manage side effects more effectively. The role of physicians in ABC treatment is more important now than in the past because these treatments require much greater medical input and time commitment than preceding therapies.
For this article, EMJ conducted a discussion on 6th March 2020 with two key opinion leaders (KOL), Prof George W. Sledge Jr from the USA and Dr Antonio Llombart-Cussac from Spain, both of whom have a wealth of experience and expertise in managing ABC, to gain their perspectives on a range of topics in this area. These experts gave valuable insights into several pertinent issues in ABC treatment and discussed significant recent developments in the field.
The article discusses the results from key clinical trials for CDK4/6 inhibitors in patients with HR+, HER2- ABC and places these drugs in the context of current therapeutic options for this disease. Treatment recommendations, strategies for treating previously difficult-to-treat patients, and clinical situations and approaches to enable patients to manage side effects are also explored.
KEY TRIALS FOR CDK4/6 INHIBITORS IN COMBINATION WITH FULVESTRANT: MONARCH 2, PALOMA 3, AND MONALEESA 3
Introduction to the Trials
The efficacy and safety of the CDK4/6 inhibitors abemaciclib, palbociclib, and ribociclib in patients with HR+, HER2- ABC have been investigated in three recent, key, randomised, double-blind, placebo-controlled, Phase III trials: MONARCH 2, PALOMA 3, and MONALEESA 3 (Table 1).
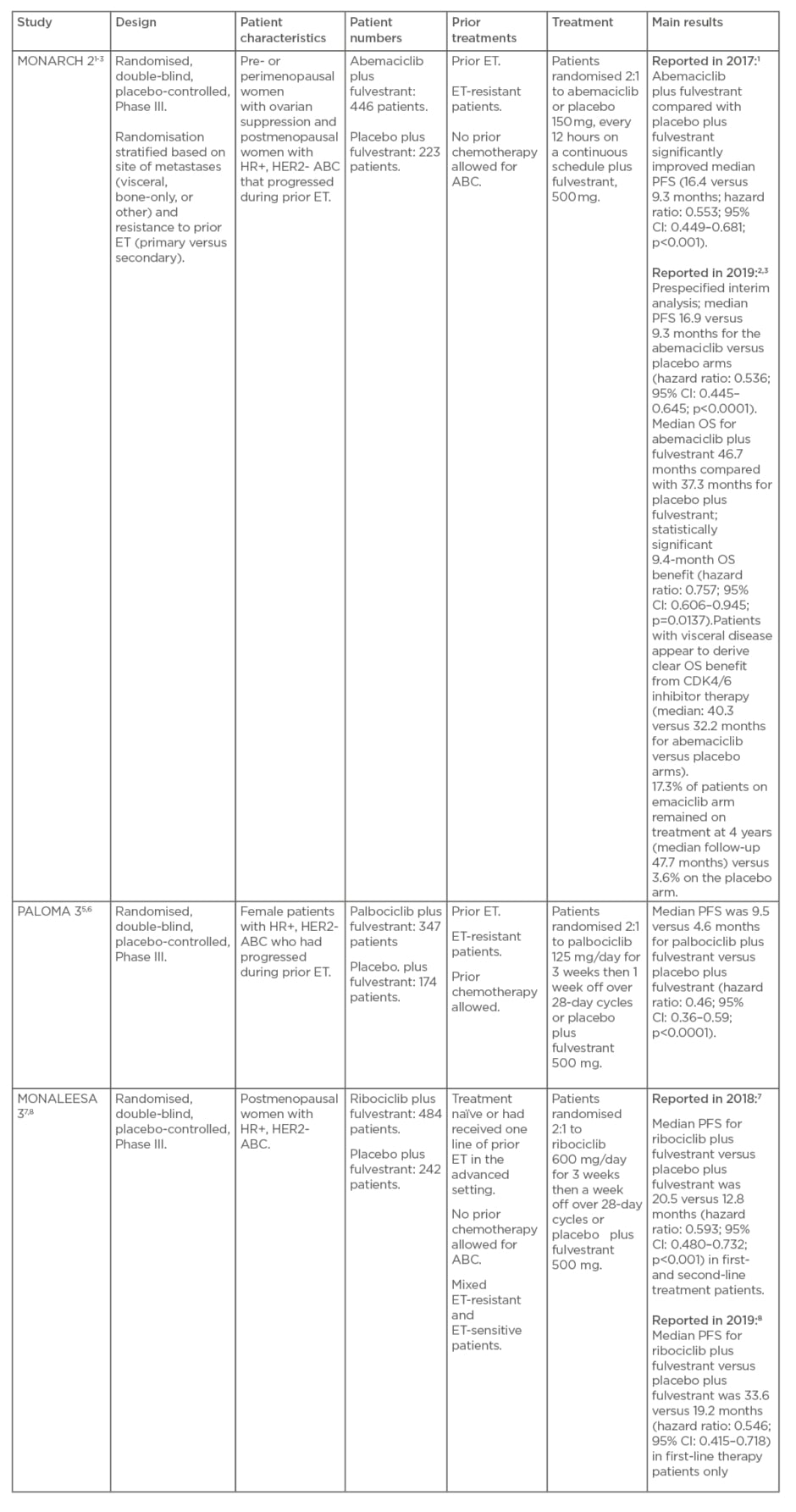
Table 1: MONARCH 2, PALOMA 3, and MONALEESA 3 studies.
ABC: advanced breast cancer; CI: confidence interval; ET: endocrine therapy; HER2-: human epidermal growth factor receptor 2-negative; HR+: hormone receptor-positive; OS: overall survival; PFS: progression-free survival
MONARCH 2 was a global trial of abemaciclib or placebo in combination with fulvestrant in pre- or perimenopausal women with ovarian suppression and postmenopausal women with HR+, HER2- ABC that progressed during prior ET.1-3 Randomisation in MONARCH 2 was stratified based on the site of metastases (visceral, bone-only, or other) and resistance to prior ET (primary versus secondary4).
In PALOMA 3, palbociclib or placebo was used in combination with fulvestrant in female patients with HR+, HER2- ABC who had progressed during prior ET.5,6
MONALEESA 3 comprised ribociclib or placebo in combination with fulvestrant as first- or second-line treatment in postmenopausal women with HR+, HER2- ABC who were treatment-naïve or had received one line of prior ET in the advanced setting.7,8
Hazard Ratios Indicate No Statistical Differences Between CDK4/6 Inhibitors
Prof Sledge explained that MONARCH 2, MONALEESA 3, and PALOMA 3 vary considerably in patient population in terms of amount of prior ET, resistance to ET, and whether there was prior chemotherapy, with MONARCH 2 and PALOMA 3 conducted in ET-resistant patients and MONALEESA 3 mixing patients who are ET-resistant and ET-sensitive. Prior chemotherapy for ABC was allowed in PALOMA 3 but not in MONARCH 2 and MONALEESA 3.
Cross-trial comparisons of these three trials are very difficult because of the different patient populations; however, Prof Sledge emphasised that it is possible to draw some confidence statistically by looking at the hazard ratios for the primary endpoint of progression-free survival (PFS).
In 2017, the MONARCH 2 authors reported that abemaciclib plus fulvestrant compared with placebo plus fulvestrant significantly improved PFS (median: 16.4 versus 9.3 months; hazard ratio: 0.553; 95% confidence interval [CI]: 0.449–0.681; p<0.001).1 Updated PFS results in 2019 were highly consistent with those of the primary analysis: median 16.9 versus 9.3 months for the abemaciclib versus placebo arms (hazard ratio: 0.536; 95% CI: 0.445–0.645; p<0.0001).3
The results from PALOMA 3 showed median PFS was 9.5 versus 4.6 months for palbociclib plus fulvestrant versus placebo plus fulvestrant (hazard ratio: 0.46; 95% CI: 0.36–0.59; p<0.0001).6 Ribociclib plus fulvestrant was shown in MONALEESA 3 to significantly improve median PFS compared with placebo plus fulvestrant as follows: 20.5 versus 12.8 months (hazard ratio: 0.593; 95% CI: 0.480–0.732; p<0.001) reported in 2018 in first- and second-line treatment patients; 33.6 versus 19.2 months (hazard ratio: 0.546; 95% CI: 0.415–0.718) reported in 2019 in first-line-treatment patients only.7,8
As shown, the hazard ratios in these trials are similar (approximately 0.5 for each inhibitor) with overlapping CI, so statistically, Prof Sledge highlighted, there is no convincing evidence from the trials that any one of these CDK4/6 inhibitors is better than either of the others (i.e., there is no difference between the different CDK4/6 inhibitors) in the first- and second-line setting.
Dr Llombart-Cussac agreed that, globally, there is indirect evidence that the three drugs behave very similarly in both first- and second-line settings, and if there are any differences it would be very hard to observe these in any head-to-head clinical trial.
Overall Survival in MONARCH 2
The updated PFS data in MONARCH 2 presented at the European Society for Medical Oncology (ESMO) Congress in 2019 were highly consistent with the results from the primary analysis 2 years earlier (hazard ratio: 0.553 in 2017 versus 0.536 in 2019). Prof Sledge explained that these results were not surprising and confirm the positive effect of abemaciclib on PFS.1,3
What was not expected, he continued, was the statistically significant 9.4-month improvement in overall survival (OS) in the ET-resistant patients with HR+, HER2- ABC who received abemaciclib plus fulvestrant combination therapy in MONARCH 2. Prof Sledge declared that he was not aware of any drug in the metastatic HR+ setting that has improved OS by 9+ months in the last 20 years and that this is a real change for the field.
All physicians involved in clinical trials use PFS as a possible surrogate marker for eventual OS benefits. Improvements in PFS, however, are not always followed by improvements in OS. Prof Sledge gave an example of this with bevacizumab in the metastatic setting, in which multiple trials showed improvement in PFS but a lack of OS benefit to complement the PFS effect and, because of this, bevacizumab is now rarely used in the metastatic setting.
A commonality of trials in the metastatic setting is that it is rare for OS benefit to outperform PFS benefit (usually PFS benefit is greater than OS benefit); therefore, Prof Sledge was pleasantly surprised with the 9+ month improvement in OS in MONARCH 2.
Dr Llombart-Cussac commented that it will be very interesting to continue to observe the OS data in this trial, and speculated that in future, there will be even greater OS benefits. He emphasised the huge number of ET-resistant patients still on treatment in the combination arm of the trial: 17.3% of patients on the abemaciclib arm remained on treatment at 4 years (median follow-up: 47.7 months) versus 3.6% on the placebo arm.3 Prof Sledge added that the tail of the curve is impressive, which indicates a good outcome for patients. In addition to the efficacy signal, these results imply that CDK4/6 inhibitors (in this case abemaciclib) can be administered safely for prolonged periods of time, which is important for transitioning the drug to the adjuvant setting.
Worse Outcome in Heavily Pretreated Patients
According to Prof Sledge, as would be expected, the trial with the most heavily pretreated patients, the PALOMA 3, is the trial with the worst patient outcomes.4,5 Prof Sledge urged not to take treatment differences observed between PALOMA 3 and MONARCH 2 as evidence that the CDK4/6 inhibitors act differently but that the populations in these trials are very different.
Dr Llombart-Cussac highlighted the strength of MONARCH 2 as the purest trial in terms of endocrine resistance definition and criteria (pure metastatic, endocrine-resistant) and with a well-defined patient population. This type of patient is more common in clinical practice, i.e., patients who have progressed on adjuvant aromatase inhibitors, so this trial demonstrates a real-life scenario and is more clinically relevant to the physician. In contrast, Dr Llombart-Cussac explained, PALOMA 3 had much broader inclusion criteria, allowing prior chemotherapy and prior ET, and the definition of endocrine resistance/sensitivity was based on prior ET but did not take into account chemotherapy received.
LOOKING FOR DIFFERENCES BETWEEN THE CDK4/6 INHIBITORS
Head-to-Head Trials for CDK4/6 Inhibitors are Unlikely
The KOL were asked if there is any requirement or interest in conducting a head-to-head trial for the CDK4/6 inhibitors. Prof Sledge expressed that although the medical oncology community would be delighted to see a head-to-head trial of the three CDK inhibitors (abemaciclib, palbociclib, and ribociclib), this may not receive the required support from the pharmaceutical industry, and head-to-head trials in the past have not been the most popular trials to recruit to. Unless there is a compelling biological explanation why one CDK4/6 inhibitor would be better than another, or compelling evidence from trials that one inhibitor was superior to another, community interest in such a trial would be low, particularly given how many other novel treatment options are currently flooding the treatment space.
Dr Llombart-Cussac gave an example of an earlier, unsuccessful, head-to-head trial programme. He explained that around 20 years ago, the oncology community enrolled more than 30,000 patients on more than 10 trials to compare the different aromatase inhibitors and this was supported by an evidence-based, statistically based rationale. Unfortunately, these trials failed and did not show a single difference between the drugs. Although patients and healthcare professionals might be interested in a head-to-head trial of CDK4/6 inhibitors, Dr Llombart-Cussac commented that there is a very low expectation that the research will be really helpful in terms of ascertaining whether one drug is superior to another.
According to Prof Sledge, there is a lot we do not know about all three CDK4/6 inhibitors, such as mechanism of resistance (do they all have the same mechanism?) and whether they work in the same way across all subpopulations. He expanded: “In the absence of a head-to-head comparison trial, a lot of what we are doing is hand-waving in terms of trying to come up with explanations for differences.”
Crossover from One CDK4/6 Inhibitor to Another
Although head-to-head trials are unlikely, Prof Sledge proposed that the oncology community could gather some interesting information if patients who progressed on one CDK4/6 inhibitor are crossed-over to receive another CDK4/6 inhibitor to assess whether this is associated with any benefit. Such a trial could be conducted in a small patient sample. A small benefit would indicate that such a crossover would not be useful in clinical practice, whereas a significant crossover benefit would be an interesting, clinically important result.
TREATMENT STRATEGIES FOR PATIENTS WITH HR+, HER2- ADVANCED BREAST CANCER AND VISCERAL DISEASE: A CHANGE IN CLINICAL PRACTICE
Visceral Disease: “Usually a Trouble Spot for Endocrine Therapy”
According to Prof Sledge in his presentation at ESMO 2019, visceral disease in patients with HR+, HER2- ABC is “usually a trouble spot for ET.” This phrase is explained by looking historically at how patients with HR+, HER2- ABC respond to treatment. There is considerable clinical trial experience indicating that patients with visceral disease, particularly liver metastasis, historically fare poorly with ET alone.
In general terms, factors for a poorer prognosis in patients with HR+, HER2- ABC are resistance to prior ET (particularly primary resistance to a primary ET) and liver metastasis; hence, the MONARCH 2 trial stratified for site of metastases and endocrine resistance.1-3
So, why do patients with liver metastases have such a poor prognosis? Prof Sledge admitted that there is not necessarily a good answer to that question, other than that historically, liver metastasis was often discovered at a fairly late and large volume stage.
Overall Survival Benefit in Patients with Visceral Disease in MONARCH 2
Analysing OS by metastatic site in MONARCH 2 shows that patients with visceral disease appear to derive clear benefit from CDK4/6 inhibitor therapy (median: 40.3 versus 32.2 months for abemaciclib versus placebo arms).3 One of the very reassuring findings from MONARCH 2, according to Prof Sledge, is that patients who are historically thought to respond poorly to treatment (those with liver metastasis or endocrine resistance) appeared to derive significant benefit with the addition of abemaciclib to fulvestrant. In addition to this simply being good, positive news for the patient, it implies that CDK4/6 inhibitors in general (and perhaps abemaciclib in particular) work in a fundamentally different way to hormone therapies in the past and this is probably why there are positive differences in previously difficult-to-treat patients. However, it is not clear whether there are any biological differences in terms of treatment effect and patient response between the different types of visceral disease (e.g., liver metastases and lung metastases).
Timing of Chemotherapy and CDK4/6 Inhibitors in Visceral Crisis
Dr Llombart-Cussac described that, historically, physicians treating breast cancer patients with endocrine criteria had to decide between chemotherapy and single-agent ET. Even though all national and international guidelines recommended ET, he noted, oncologists were a little reluctant to follow these guidelines because they were concerned that ET was a palliative treatment and more benefit would be obtained with chemotherapy.
This led to the evolution of concepts such as visceral crisis, which is a very political criterion to define. It represents a clinical scenario that is impossible to characterise using clinical criteria and is defined by the perception of the physician. Prof Sledge agreed that visceral crisis is problematic in terms of the definition, as physicians report: “I know it when I see it;” it is therefore very hard to define visceral crisis.
Large-scale liver involvement is a key component of visceral crisis and for many physicians, liver involvement was, and still is, an easily prescribed criterion for chemotherapy. For example, if Prof Sledge saw a patient with 75% of the liver gone, an ER of 8%, and who was progesterone-receptor-negative, that patient would probably be administered chemotherapy, although this is an uncommon scenario.
Dr Llombart-Cussac clarified that in patients with visceral crisis, the physician also needs to take into account the endocrine sensitivity potential of the tumour, and he would consider chemotherapy for a patient even without visceral disease if the patient progressed within 6 months of ET and had an aggressive profile. Current clinical practice has therefore introduced factors that were not considered in the past when addressing patients with visceral crisis; aside from factors relating to patient characteristics, the physician must consider tumour biological characteristics.
Although MONARCH 2 does not compare abemaciclib with chemotherapy, observing the survival benefit with abemaciclib in patients with visceral disease is very encouraging considering there is no reported survival benefit in favour of chemotherapy in this scenario.
Administering CDK4/6 Inhibitors Before Chemotherapy in Patients with Visceral Crisis
Many physicians believe chemotherapy to be a very good option in patients with endocrine-resistant ABC. However, the OS benefit with CDK4/6 inhibitors in patients with visceral disease indicates that such patients should be treated with CDK4/6 inhibitors first, i.e., before chemotherapy, in clinical practice. The interesting findings in MONARCH 2 are helping to inform physicians that patients should receive a CDK4/6 inhibitor (in this case abemaciclib) first and that chemotherapy should be delayed even in cases of endocrine resistance and visceral metastases.
Dr Llombart-Cussac clarified that at this point, the only relevant question concerning the timelines for chemotherapy and CDK4/6 inhibitors is for the patients considered to be in visceral crisis and this is a scenario for which a clinical trial cannot be planned because of the characteristics of this population.
Although the guidelines recommend chemotherapy as initial treatment, there is a drug combination (ET and CDK4/6 inhibitors) that is more active in terms of response and less toxic than chemotherapy and it is hard to continue to say that in HR+, HER2- ABC and visceral crisis chemotherapy should be the standard. It is not possible to build evidence for use of CDK4/6 inhibitors ahead of chemotherapy in these patients based on clinical trials, but data generated from real-life cases will help to clearly identify if these patients should receive ET and CDK4/6 inhibitors, with their lower toxicity, higher effectiveness, and major patient benefits, before chemotherapy.
Prof Sledge concurred with this expectation and shared that this is an exciting potential development with this novel approach. He referred to an Eastern Cooperative Oncology Group (ECOG) trial of E1193 in the 1990s in which a median PFS of 6 months was reported for paclitaxel administered as frontline therapy for metastatic disease. 9 Although this was not a direct comparison trial, Prof Sledge indicated that abemaciclib appears to be better than an active chemotherapy drug in terms of preventing progression and the fact that such improvement is seen in previously difficult-to-treat subsets of patients (those with visceral disease or endocrine resistance) is very reassuring for this class of drugs.
Is Abemaciclib Representative of CDK4/6 Inhibitors for Patients with Visceral Disease?
The beneficial results for abemaciclib in patients with HR+, HER2- ABC and visceral metastasis in MONARCH 2 may be representative of those for palbociclib and ribociclib; however, as highlighted by Prof Sledge, there are not sufficient data to show abemaciclib is superior to other CDK4/6 inhibitors for visceral disease, although this is more from a lack of data with the other CDK4/6 inhibitors.
In terms of site of metastases (visceral, bone-only, or other) and endocrine resistance, Dr Llombart-Cussac explained, MONARCH 2 provides robust data for abemaciclib because these two criteria were set as stratification factors, thus generating strong confidence in the data for physicians.1-3 In contrast, PALOMA 3 and MONALEESA 3, with their broad patient characteristics, did not show the same trends. Prof Sledge added that in PALOMA 3, the clinical benefits appeared to be greatest in patients who were less endocrine insensitive.6 He conjectured that if there is a difference between the CDK4/6 inhibitors it may be manifesting in terms of the amount of prior ET or ET resistance but referenced once more the limitations of cross-trial comparisons.
BONE-ONLY DISEASE
During his presentation at ESMO 2019, Prof Sledge mentioned that bone-only disease data were not yet mature for MONARCH 2 and he confirmed at the time of discussion that the data are still premature.2 Prof Sledge explained that survival data tend to mature later for bone-only patients compared with patients with lung or liver metastasis. Patients with bone-only disease tend to fare better than those with visceral disease and when asked if he expected to see this in MONARCH 2, Prof Sledge indicated that he would certainly expect this in the control population (placebo plus fulvestrant). Historically in HR+, HER2- ABC clinical trials, he continued, patients with bone-only disease have frequently done better, and it is not unusual to see a patient with a single bone metastasis do well for years on ET.
DIFFERENCES IN CDK4/6 INHIBITOR TREATMENT SCHEDULE
According to Dr Llombart-Cussac, the differences between abemaciclib and the CDK4/6 inhibitors palbociclib and ribociclib are based mostly on preclinical data and differences in treatment schedule. Abemaciclib is administered on a continuous schedule, with no need to interrupt treatment, whereas palbociclib and ribociclib are administered for 3 weeks, followed by 1 week off. This difference could be a clear advantage for abemaciclib. Dr Llombart-Cussac advocated administration of abemaciclib to patients with more aggressive scenarios and explained this was justified to avoid nonexposure of the tumour to a CDK4/6 inhibitor for 1 week, particularly because increases in Ki67 protein measurements have been observed when treatment is stopped.
Unsurprisingly, a continuous schedule of treatment leads to a different toxicity profile; because patients are continuously exposed to CDK4/6 inhibitors there are more side effects, such as diarrhoea and abdominal pain. Prof Sledge stated that the toxicity difference between a continuous and an interrupted drug schedule is notable and given the discomfort associated with diarrhoea, this is an important issue for patients. He also mentioned that preclinical data indicate there is some difference in terms of relative inhibition between CDK4 and CDK6 (e.g., abemaciclib is 14-times more potent against CDK4 than CDK6 in enzymatic assays10) but it is unknown whether these differences in relative potency are important in the clinic.
MANAGING SIDE EFFECTS THROUGH ACTIVE MANAGEMENT AND PATIENT EDUCATION
There are great potential benefits with the CDK4/6 inhibitors; however, toxicity of these drugs is a major concern for patients (Table 2).11,12 Dr Llombart-Cussac explained that toxicity is the major difference between abemaciclib and the other CDK4/6 inhibitors and that there is a learning curve associated with the use of abemaciclib. In contrast to palbociclib and ribociclib, patients need to be educated on how to manage side effects such as diarrhoea with abemaciclib treatment. Information on tolerating loperamide (to decrease diarrhoea frequency) and advice on diet are two examples of the education strategy managed by physicians and nurses. These healthcare professionals need to gather experience and use that experience to educate patients to manage side effects.
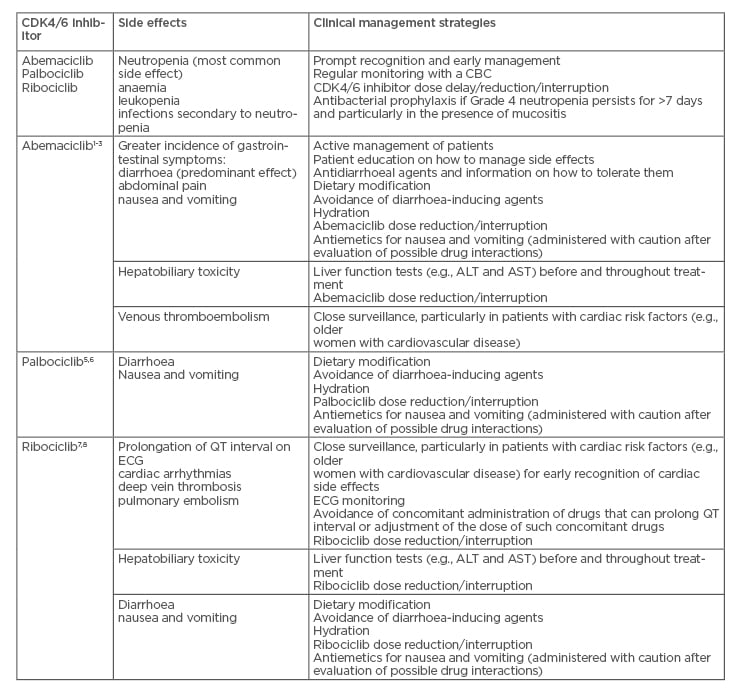
Table 2: Side effects of the CDK4/6 inhibitors abemaciclib, palbociclib, and ribociclib.11,12
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CBC: complete blood count; CDK4/6: cyclin-dependent kinase 4 and 6; ECG: electrocardiogram.
The toxicity profile of abemaciclib is likely to improve in the future simply through the increasing experience of physicians, nurses, and clinical trial investigators with the drug. Dr Llombart-Cussac predicted that the problems with diarrhoea he observes in his clinic will decrease and could become almost nonexistent. In his experience, educating patients about the side effects of abemaciclib and how to manage them has decreased reporting of Grade 3 and Grade 2 diarrhoea and he indicated that training and education need to improve in the future. Toxicity is a major issue of CDK4/6 inhibitors but is reduced by physician experience, active management, and patient education.
Prof Sledge explained that comparing ET alone with ET plus CDK4/6 inhibitors highlights one of the major differences for patients and the physicians and nurses caring for them: the requirement for active management and patient education. The CDK4/6 inhibitors form a new class of drugs with great potential benefits that have been a major positive for patients; however, they necessitate much more time input from physicians and nurses in terms of patient care than previous treatments. Historically, physicians could prescribe aromatase inhibitors to patients and ask them to come back for a check-up in 3 months.
The situation with CDK4/6 inhibitors is very different: administration of these drugs requires time commitment from healthcare professionals and patients alike.
STANDARD-OF-CARE IN HR+, HER2- ADVANCED BREAST CANCER
According to Dent13 in her presentation at ESMO 2019, the results from MONARCH 2 and MONALEESA 3 solidify CDK4/6 inhibitors in the metastatic setting.
Prof Sledge agreed that given the continuing PFS benefits seen in all the CDK4/6 inhibitor trials and the emerging OS benefits observed, these inhibitors now represent standard-of-care in HR+, HER2- ABC. He confirmed that these trial results led to a change in clinical practice because the use of these agents is now part of clinical guidelines and they are standard-of-care in the USA.
Dr Llombart-Cussac verified that this was also the case in Europe, where CDK4/6 inhibitors have been introduced in the market in ET-resistant and ET-sensitive scenarios. He clarified that the use of CDK4/6 inhibitors as standard-of-care is mostly based on regulatory approval, not on the confidence of physicians in the data. These inhibitors become the preferred treatment for more than 70% of the population as soon as the regulatory authority in each country approves them for different scenarios and combinations. CDK4/6 inhibitors are considered standard-of-care for countries including Spain, Italy, France, and Germany and are not standard-of-care only in countries where there is no reimbursement.
CONCLUSIONS
The insights of the two KOL in this article clearly show that the CDK4/6 inhibitors are changing and improving the treatment of ABC, including in previously difficult-to-treat subpopulations. As physicians, nurses, and other healthcare professionals gain knowledge and experience of these inhibitors, and engage in active management of their patients, this will lead to better education of patients and, in turn, improve patient management of side effects. Recent clinical trial data indicate significant PFS and OS benefits with CDK4/6 inhibitors, with no statistical evidence of superiority within the drug class. Notable OS benefits in patients with visceral disease are a particularly exciting development in this field and indicate that changes to conventional treatment strategies should be considered to administer CDK4/6 inhibitors before chemotherapy in visceral crisis. First-line administration of CDK4/6 inhibitors not only extends the length of time a patient with ABC can use ET but may also improve quality of life in terms of postponing the need to initiate the more toxic chemotherapy. There is so much more to learn about the CDK4/6 inhibitors but continued follow-up of current clinical trials, initiation of new clinical trials, and collection of data generated from real-life cases will all contribute to the quest.
Biographies
Prof George W Sledge Jr
Professor and Chief of Medical Oncology, Stanford University School of Medicine, Stanford, California, USA
Prof Sledge specialises in the study and treatment of breast cancer and directed the first large, nationwide study on the use of paclitaxel to treat advanced breast cancer. His recent research focusses on novel biological treatments for breast cancer. He has published over 370 scientific articles spanning both laboratory and clinical research.
Prof Sledge is Past President of the American Society of Clinical Oncology (ASCO). He was chair of the Breast Committee of the Eastern Cooperative Oncology Group (ECOG) from 2002 to 2009, where he led the development of nationwide clinical trials. Prof Sledge was awarded the Hope Funds for Cancer Research 2013 Award of ‘Excellence for Medicine’ and was the recipient of the 2006 Brinker Award for Scientific Distinction, the 2007 Breast Cancer Research Foundation’s Jill Rose Award, and the 2010 William L. McGuire Award from the San Antonio Breast Cancer Symposium.
Dr Antonio Llombart-Cussac
Medical Oncology Department Manager, Hospital Arnau de Vilanova, Valencia, Spain
Dr Llombart-Cussac is internationally renowned as a breast cancer expert, has published more than 150 articles in international journals, and participates in research projects with competitive grants both in Spain and internationally.
Dr Llombart-Cussac is coordinator of the IRB-Lleida Breast Cancer Research Group, Associate Professor at the University of Lleida, Lleida, Spain, and Scientific Coordinator of the SOLTI Breast Cancer Research Group. He is also a member of the main European and American societies for oncology. Dr Llombart-Cussac studied at the Universities of Valencia and Navarra and undertook his residency at Valencia’s Clinical Hospital. He visited the Institut Gustave Roussy, Villejuif, France, from 1994 to 1997 and Memorial Sloan Kettering Cancer Center, New York City, New York, USA, in 2001. He practised at the Valencian Oncology Institute Foundation from 1998 to 2005 and was Medical Oncology Department Manager at Arnau de Vilanova Hospital in Lleida from 2005 to 2011.

![EMJ Oncology 8 [Supplement 2] 2020](https://www.emjreviews.com/wp-content/uploads/2020/08/EMJ-Oncology-8-Supplement-2-2020-Feature-Image-940x563.jpg)






