Abstract
Accessory breast is a congenital atavism condition. Accessory breast tissue may arise anywhere along the mammary line because of the failure of complete maturation during embryogenesis. The malignancy in accessory breast tissue is considered as primary breast cancer.
Axillary breast cancer is an under-recognised site of primary breast cancer. The authors presented a case report of a 52-year-old premenopausal female who presented with axillary immobile mass in her left axilla and who was diagnosed after extensive investigations with Stage II B oestrogen receptor (ER)/progesterone receptor (PR) positive, human epidermal growth factor 2/neu proto-oncogene (HER2/neu) negative, and poorly differentiated accessory breast adenocarcinoma. The patient was designated as Stage II B, and following the 2012 National Comprehensive Cancer Network (NCCN) guidelines for breast cancer management, was surgically treated, followed by postoperative adjuvant chemotherapy in the form of four cycles of doxorubicin and cyclophosphamide (AC protocol), and then four cycles of docetaxel. Subsequently, radiotherapy was given followed by hormone therapy. The patient was followed up for 7 years, and at the time of publication, is alive and stable.
Accessory breast cancer is a rare disease and misdiagnosis of these cases is a common problem, leading to extensive and unnecessary investigations; therefore, physicians must be aware of these cases. Management of accessory breast cancer is according to the same guidelines provided for management of the condition. Follow-up data should extensively encourage the determination of the prognosis of accessory breast cancer in comparison to common breast cancer.
INTRODUCTION
Accessory breast cancer is a rare disease developed from accessory breast tissue. Accessory breast tissue can be found along any point of the mammary lines, including in the thoracic and abdominal region (67%) and as low as the groin. Ectopic breast tissue can also be found in locations such as the face, back, and thighs, but the predominant site is the axilla. The incidence of supernumerary breast and ectopic breast tissue around the world is 1–6%.1 It affects 2–6% of females and 1–3% of males, but there is no definite incidence number for accessory breast malignancy; furthermore, reports for accessory breast cancer only include case reports and case series. Occurrence rates differ extensively according to ethnicity and gender, ranging from as low as 0.6% in Caucasians (relatively common among Asian women) to as high as 5% in Japanese females and Native American populations.2,3 The 1915 Kajava classification system classified accessory breasts as Class I–VIII according to anatomical structure, and is still used today (Table 1).4,5
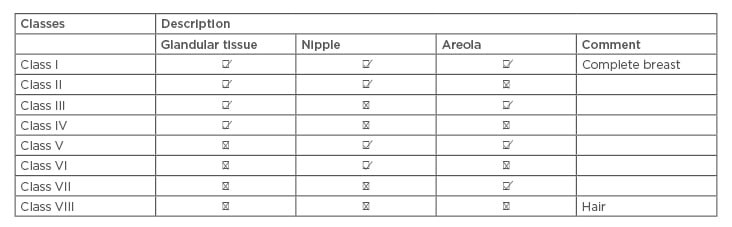
Table 1: Kajava classification 1915.
Accessory breast tissue can be located along the chest wall, vulva, axilla, knee, lateral thigh, buttocks, face, ear, and neck. Changes or symptoms may be noticed during puberty, at different times of the menstrual cycle, or during pregnancy and breastfeeding in women. Of note, the accessory breast tissue is often not detected until puberty because it is activated by hormones.6
CASE REPORT
A 52-year-old premenopausal female with diabetes, a blood pressure of 130/70 mmHg, a weight of 76 kg, a height of 167 cm, and no family history of breast cancer presented to the general surgery outpatient department on the 4th of December 2011. The patient had a history of left axillary immobile mass of around 3.0×2.0 cm (length/width) which had increased in size within the prior 6 months, but had no history of a lump in the breast or discharge from the nipple. The patient mentioned that this axillary mass had been present since puberty but had been gradually increasing in size. The patient had a negative family history of malignancy and ultrasound (US) breast screening was performed which showed no mass in the breast. Multiple lymph nodes were seen, however, in the left axilla, with the largest lymph node being 4.4×3.5 cm.
On the 17th of December 2011, tissue biopsy was taken from the left axillary mass which measured 4.5 cm in maximum diameter, weighed 30 g, and to which bio-section showed a
greyish-white solid cut surface with pinkish and yellowish areas. Four blocks representing the whole cross-section of the tumour were embedded in three cassettes. Microscopically, the sections showed lobules of a malignant epithelial tumour involving the subcutaneous adipose tissue, dermis, and reaching up to the overlying epidermis. It showed large areas of partial or complete ischaemic necrosis, scattered mitotic figures, and tumour cells arranged in solid sheets or lobules without glandular differentiation (Figure 1A). Immunohistochemistry (IHC) methodology reported that the tumour cells stained positively for pan-cytokeratins (CK) (AE1/AE3), suggestive of lymph node micrometastases;7 CK7; epithelial membrane antigen; ER, that formed 50% of tumour cells; and PR, that formed 5% of tumour cells (Figure 1B).
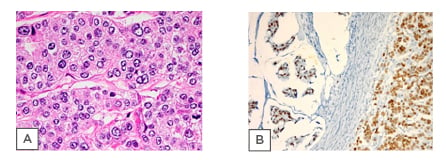
Figure 1: A) Histological examination of specimen. Sections show large deposits of carcinoma replacing most of the lymph node structure. B) The tumour cells in both mucinous and nonmucinous areas show a positive staining for oestrogen receptor.
IHC results were not exclusively positive; HER2, BerEP4 (histologic stain used to aid in the diagnosis of basal cell carcinoma), mammoglobulin (highly specific for most breast cancers), CK5/6 (aid to differentiate between mesothelioma and other forms of cancer), thyroid transcription factor-1 (sensitive marker for pulmonary and thyroid adenocarcinomas), CD10 (sensitive immunohistochemical marker of normal endometrial stroma), smooth muscle actin (aid in diagnosis of ovarian carcinoma), S100 (common marker of neural tissue/lesions and melanoma), chromogranin (aid in diagnosis of carcinoid tumours), synaptophysin (neuroendocrine marker), CK20, p63 (rules out invasion in breast tumours by determining presence of myoepithelial cells), and p53 all showed a negative reaction. Therefore, the possibilities of neuroendocrine, primary lung, primary renal tumours, among others were not supported by the IHC results. Reports showed moderate to poorly differentiated adenocarcinoma in favour of primary breast cancer (Table 2).
On the 26th February 2012, the patient was referred to medical oncologists for further management and workup. Chest, abdomen, and pelvis CT scans were performed to rule out any primary sites, which all came back negative, and only the left axillary lymph node was seen to be involved. Bone scan results, as well as upper gastrointestinal endoscopy and colonoscopy results investigating for primary tumours, were also negative, and there were no signs of any malignancy. MRI of the breast revealed no pathology apart from multiple axillary lymph nodes. Tumour markers; cancer antigen-breast (CA 15-3) was high at 107.7 and CA 125 was normal (Table 2).
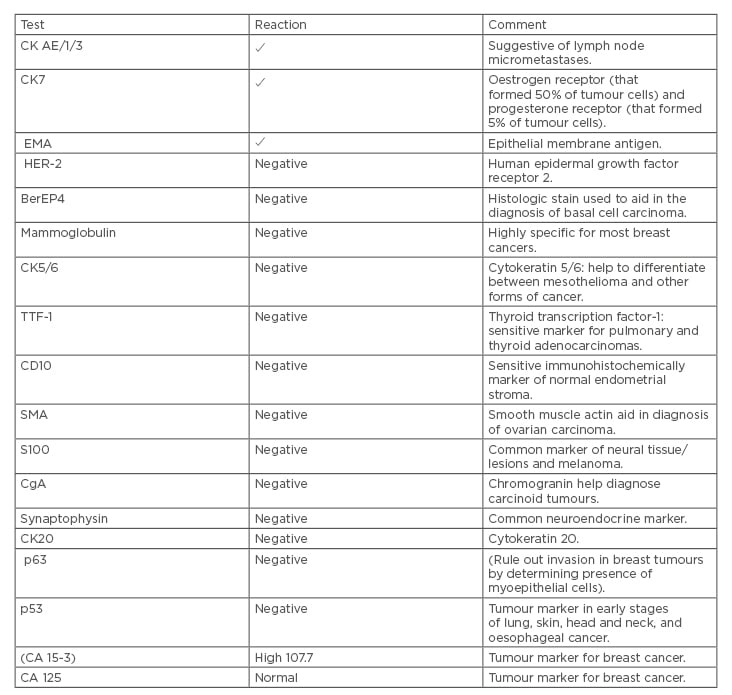
Table 2: Immunohistochemistry test results.
In April 2012, she underwent left axillary clearance and the histopathology report confirmed primary breast adenocarcinoma.
On the 19th of May 2012, the patient received chemotherapy; AC protocol doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 21 days for 4 cycles, followed by 4 cycles of docetaxel 100 mg/m2. Every cycle was 21 days and the patient tolerated it well. The patient then underwent radiotherapy and hormonal therapy (tamoxifen 20 mg oral daily). She underwent follow-up every 6 months until present. To assess the prognosis of the case, the Nottingham prognostic index for breast cancer was used. It gave an index value of 4.6, placing it in the moderate group, with 5 year survival.8 The National Health Service (NHS) Predict tool was applied as well for prognostic assessment, including hormonal status, and showed a result of 10 years survival.9 In 2019, after 7 years of starting the treatment, the patient is doing well, free from any signs of malignancy, recurrence, or complications. The mammography (MMG) results were negative.
DISCUSSION
Accessory breast is a congenital condition when accessory normal breast tissue is present at abnormal sites.10 Accessory breast is also known as polymastia, supernumerary breasts, auxiliary breast, ectopic breast, adnexal, or mammae erraticae. These types of breasts sometimes appear to have or lack nipples or areolae, making them ambiguous.11
In Caucasians, the incidence of ectopic breast tissue is 1–4%, while it is more frequent in the Far East, especially Japan, among both genders. Its incidence rate was evaluated as 5.19% in Japanese women, and as 1.68% in Japanese men with hereditary factor.12 These abnormal tissues are most frequently seen in the axilla, followed by the area just lower and in half of the cases this abnormality is shown on both sides.12 In rare cases it was reported in some other areas including the acromial or scapular region, vulva, and in the midline of the thorax and abdomen.13 Ectopic breasts enlarge during pregnancy and lactation and may lactate if they have a working ductal system. Breastfeeding from ectopic breasts was reported.12 Disorders of breast tissue such as adenofibroma, cysts, and carcinomas have also been reported in ectopic breasts similar to normal breast tissue and carcinomas are rare.14-16 The types of carcinoma seen within the ectopic breast tissue include ductal, lobular, mucinous, medullary, papillary, and invasive secretory (juvenile) carcinomas.13,15,16
Ectopic breast tissue develops embryologically because of failed resolution of the mammary ridge or milk line. An ectodermal tissue extends from the axilla to the inguinal folds and shows in the 6th week of gestation. The axilla is the most frequent site followed by the area inferior to the normal breast and may appear anywhere along the milk line.17
Hormonal influences affect ectopic breast tissue, just as they would in a healthy breast, and can develop similar types of benign and malignant disorders. Fibroadenomas, cysts, duct hyperplasia, and infrequently carcinoma can arise.18,19 The incidence of accessory breast cancer as per reports is between 0.3% and 0.6% of all breast cancers.19
Ectopic primary breast cancer in the axilla comprises 60–70% of all cases reported.20 Differential diagnosis of the accessory breast cancer includes many disorders. In the axillary area, it can be confused with lipoma, lymphoma, lymphadenitis, metastatic lymphadenopathy, sebaceous cyst, and hidradenitis suppurativa. MMG and US of the breast can aid the exclusion of other breast pathologies. US can also detect ectopic breast tissue as an echogenic area resembling normal glandular tissue, as well as detect characteristics of the mass. MMG cannot capture ectopic breasts because of their peculiar location, but it can be visualised in the axilla by oblique and exaggerated craniocaudal views.21
Cancer of ectopic breasts is depicted as a typical malignant mass with the same characteristics of those of metastatic axillary lymph nodes associated with malignant tumour. No specific findings for accessory breast cancer are detected.21 Using MRI, the signal intensity of ectopic breast tissue is similar to that of the adjacent breast tissue, but with variability of the amount of interspersed fat. Pathological confirmation through fine-needle aspiration cytology or Tru-cut® biopsy of the mass should be performed to harvest suspect cells or tissue. Invasive ductal carcinoma, such as in traditional breast cancer, is the most common histological type with 79% of all accessory breast cancer.22
Lobular, mucinous, medullary, apocrine, and papillary carcinomas are detected in these cases and cystosarcoma phyllodes are also described. In 2011, Nihon-Yanagi et al.20 found that medullary, mucinous, and apocrine carcinomas were more common among accessory breast cancer for unknown reasons.
Regarding the management, surgical interference of accessory breast cancer combines wide resection of the tumour with surrounding tissue, skin, and axillary lymph nodes dissection.18 Ipsilateral mastectomy has no additional benefit regarding survival considering that MMG and US of the anatomic breast are normal, as was in the present case, but should be performed when differential diagnosis is challenging in some situations.18
In 2015, Zhang et al.18 recommended mastectomy if the accessory breast is closely connected to normal breast tissue, otherwise it is unnecessary.
The same regimens and protocols for postoperative treatment are used for anatomic breast carcinoma. Radiotherapy of the tumour site is recommended to control local spread, and radiation of the ipsilateral anatomic breast is not usually performed. Adjuvant therapy is mostly required because lymph node disease is usually also present. The prognosis of accessory breast cancer is difficult to assess due to limited follow-up and staging data as well as small sample size. Some authors have reported worse outcomes of accessory breast cancer than other anatomic breast cancer as the tumour is near the axillary lymph nodes and is therefore exposed to early metastasis to these nodes.18
In a report in 2011, 94 Japanese cases were reviewed and indicated that accessory breast cancer has a higher risk of lymph node metastasis than usual breast cancer.20
Accessory breast tissue is more disposed to malignant change than normal breast tissue. No definite number is reported for the incidence of accessory breast cancer among population.23 Accessory breast cancer patients experience clinical presentations as swelling, thickening, tenderness, irritation, and sometimes limited motion of shoulder. These symptoms are commonly exacerbated at the onset of puberty and pregnancy.24 Accessory breast cancer is a rare entity and has a substantial chance for misdiagnosis especially when there is an absence of anatomical breast structure such as the nipple and areola.25 The accessory breast may also not show up; therefore, MRI can be used to differentiate it from the normal breast tissue.3
The diagnosis as well as symptoms of accessory breast carcinoma are the same as for breast carcinoma,16 except for some differences including excess axillary fat, lymphadenitis, lymphoma, metastatic carcinoma, and hidradenitis suppurativa.6 Accessory breast cancer is diagnosed as breast carcinoma using MMG and US, followed by pathologic diagnosis by fine-needle aspiration cytology or core biopsy.26
The MMG is an effective tool for the assessment of breast carcinoma but not for accessory breast cancer assessment. Core tissue biopsy especially when accompanied by IHC is a very effective tool for early diagnosis and treatment of accessory breast carcinoma.2
The presented case was very perplexing because it was a rare condition for primary axillary breast cancer. The lack of areola and nipple (according to Kajava classification 1915 this case classified as Class IV) made the clinical diagnosis very difficult, confounded by the IHC results being inconclusive. This lead to extensive and unnecessary lab tests and investigations. The possibility of the normal breast being the primary site was not supported by negative staining of mammoglobulin, gross cystic disease fluid protein. Furthermore, IHC did not support the possibilities of a neuroendocrine tumour or the lung, and the kidney as the primary sites.
In the beginning the case was suspected to be a metastatic adenocarcinoma with small components of mucinous carcinoma; however, the lymph nodes were positive (13/15) with large deposits of carcinoma replacing most of the lymph node structure favouring primary breast including the possibility of axillary (accessory) primary breast. The possibilities included breast, and to a lesser extent, primary adnexal tumour. Oncologists were not satisfied about histopathology results, and so all thoughts were redirected towards it being a secondary tumour from the breast or other sites. As such, more investigations were requested including CT, MRI, CA 125, and upper gastrointestinal endoscopy, but all results were negative for the detection of unknown primary tumours.
A Tumour board committee was held to discuss this case and it was agreed that it is a primary accessory breast tumour because no original tumour could be detected in the breast, adnexa, colon, or lung. The histopathology was in favour of primary breast because CA 15-3 high and ER and PR were positive as well as previous case reports and literature review for similar cases.
The patient was staged as Stage IIB, ER/PR positive, and HER2/neu negative poorly differentiated adenocarcinoma.
The patient was treated with common breast carcinoma methods following NCCN guideline 201227 by surgery followed by chemotherapy. This included 4 cycles of AC and 4 cycles of docetaxel followed by a 50 g radiotherapy and tamoxifen for 5 years, and cancer antigen (CA 15-3) showed a significant decline after treatment (Figure 1).
Prognosis of accessory breast cancer is difficult to institute because of the absence or limited follow-up data as well as the small sample size,28 in the follow-up case the patient is stable with very good performance status for 7 years and without any complications.
CONCLUSION
Accessory breast cancer is an uncommon type of cancer and the incidence of it has no definite number. The diagnosis of these cases is challenging, and misdiagnosis will lead to extensive and unnecessary investigations. Despite the fact that carcinoma arising in axilla as a primary site is a rare condition, still the possibility of accessory breast cancer should still be considered. Management of accessory breast cancer should follow the same guidelines for breast cancer. It is important to encourage follow-up data for these cases to establish the prognosis of accessory breast cancer comparing breast carcinoma.








