Abstract
Pancreatic ductal adenocarcinoma is a lethal disease for a multitude of reasons, including difficulty of early detection, early metastatic spread, and absence of more effective therapies. Even with the advent of newer systemic therapies, the 1-year survival for metastatic disease ranges from 17–23% and 5-year survival is <5%. This necessitates an urgent need for the development of more effective modalities for early detection, particularly due to the long latent period between the genomic cellular changes and the development of metastatic disease. Currently available biochemical and molecular markers have significant potential; however, they require further clinical validation. Endoscopic ultrasound is one of the most sensitive modalities used to both screen and sample lesions, but is limited to use in high-risk patients due to its invasive nature and associated risks. Although clinically meaningful progress has been made in screening the high-risk cohorts in terms of detection of pancreatic ductal adenocarcinoma, intraductal papillary mucinous neoplasms, and mucinous cystic neoplasms, leading to early diagnosis and treatment, nonselective population-based screening is not yet available for widespread use. Currently there is no consensus on the most appropriate screening protocol for early pancreatic cancer detection. In this review, we focus on understanding the potential role of molecular and radiogenomic markers in the early detection of pancreatic cancer.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a malignancy that originates in exocrine pancreatic cells and accounts for about 95% of pancreatic cancers (PC). Despite a low incidence of around 3%, the mortality rate in the general population1 is also close to this figure, thus making PDAC a deadly disease. It was estimated that there would be 53,670 new cases of PDAC diagnosed in the USA during 2017, with approximately 43,090 deaths resulting from the disease, making it the fourth leading cause of cancer-related deaths. It is also estimated that by 2030, PC will be the second leading cause of cancer-related deaths in the USA.2
In the last decade, significant improvements have been made in the screening and therapy of multiple solid tumours, thus increasing the incremental chance for a cure in some of these cancer types. Despite these improvements, PDAC remains difficult to treat. More than 80% of PDAC cases are metastatic at the time of diagnosis, with a mean overall survival (mOS) between 3 and 6 months. Even with early diagnosis, the median survival is poor, ranging from 31.50% at 3 years to 11.86% at 5 years.3
Numerous clinical trials since the 1990s have minimally improved the mOS of patients with PDAC. Gemcitabine, a nucleoside analogue and the current standard treatment of PDAC, was tested against 5-fluorouracil in the 1990s and had an improved clinical response (24% versus 5%), mOS (5.6 versus 4.4 months), and 1-year survival (18% versus 2%);4 these outcomes occurred despite the lack of objective response.4 Other agents combined with gemcitabine have also been tested in advanced PC; however, despite higher objective response rates in some studies,5 33 randomised Phase III trials failed to demonstrate a survival benefit, with the exception of addition of erlotinib.6 FOLFIRINOX as a single agent also improved mOS by 4.3 months over gemcitabine alone (11.1 versus 6.8 months; hazard ratio: 0.57; p<0.001).7 In the MPACT Phase III trial, nab-paclitaxel plus gemcitabine, administered for the initial 3 weeks of a 4-week period, also prolonged mOS over gemcitabine alone (8.5 versus 6.7 months; p<0.001).8 These results have prompted additional trials using backbones of FOLFIRINOX or nab-paclitaxel with mixed results.9 Nevertheless, even with the advent of newer combination systemic therapies, the 5-year survival of patients with advanced disease is 8.2%, approximately a 3% improvement from 1975.3 This is, in part, caused by the lack of visible and distinctive symptoms and reliable biomarkers for early diagnosis, as well as the aggressive nature of metastatic disease with poor response to treatments.
The poor response to current therapy emphasises the need for novel and effective strategies for early screening and diagnosis, such that more patients can undergo curative surgery. This is supported by the better mOS of patients with pancreatic adenocarcinomas discovered incidentally compared to those diagnosed based on clinical symptoms (30 versus 21 months; p=0.01),10 as well as the significantly decreased complication rate of pancreatoduodenectomies, from 25–30% in the 1960s11 to as low as 2.5% in high-volume centres with expertise.12 Therefore, early screening can lead to better improvements in survival, as noted with colon and breast cancer patients, and an ideal screening strategy will have a higher sensitivity and a higher positive predictive value. Although several studies have been performed for both the asymptomatic and high-risk subject groups, the results have not been encouraging. In this review, we provide a broad description of the risk factors, currently available options for screening, and novel strategies that are presently being undertaken to improve outcomes for PDAC patients.
RISK FACTORS
Constitutional or Environmental
There are multiple constitutional and environmental factors that may influence the development of PDAC; the most common of these are listed in Table 1.
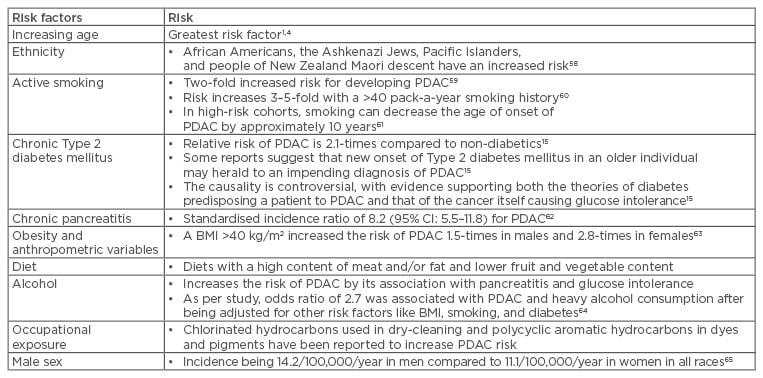
Table 1: Risk factors associated with pancreatic ductal adenocarcinoma.
CI: confidence interval; PDAC: pancreatic ductal adenocarcinoma.
Genetic Syndromes
Many syndromes that occur in multiple family members carry an increased risk of developing PDAC and are a cause of ≤17% of all PC cases.13 The risk for developing PDAC in these families is synergistically increased with environmental risk factors, such as smoking and alcohol use.14,15 These syndromes increase the patient’s life-time risk for PDAC by ≤40% with an odds ratio of ≤61.14 The syndromes with most published literature are hereditary pancreatitis, cystic fibrosis, Peutz-Jeghers syndrome, familial atypical multiple mole melanoma, hereditary breast and ovarian cancer, hereditary non-polyposis colorectal cancer or Lynch syndrome, familial adenomatous polyposis, familial pancreatic cancer, Li fraumeni syndrome, and ataxia telangiectasia, with some of them increasing the risks of other cancers as well. Families with ≥2 first-degree relatives who have PDAC, which is not associated with a known cancer syndrome, are categorised as familial pancreatic cancer. They comprise of 80% of patients with an inherited predisposition16 and BRCA2 mutations were found in 17.2% of families with ≥3 relatives with PDAC.17
Precancerous Lesions
One of the most common theories on cancer progression is the development of multiple mutations that progressively lead to invasive behaviour.18 The adenoma-carcinoma sequence is an instance of this theory and has worked well for both colon and breast cancers, for which screening has resulted in a dramatic improvement in survival. In the pancreas, there are several lesions that demonstrate slow growth and are amenable for surveillance, including pancreatic intra-epithelial neoplasia (PanIN), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasm (IPMN).
These slowly progressing lesions allow clinicians time for screening and surveillance before the lesion worsens. PanIN is the prototype of the adenoma-carcinoma sequence in the pancreas, with cells progressing from atypia, dysplasia, and then to carcinoma in situ. MCN is an epithelial neoplasm containing ovarian stroma that produces mucin but does not communicate with the pancreatic duct;19 this lesion is more likely to be invasive in older patients and has nonspecific presentations.19 Unlike MCN, IPMN are of ductal origin and produce copious amounts of mucin, which results in dilated pancreatic ducts.20 The prevalence of cancer ranges from 57–92% in main-duct IPMN and 6–46% in branch-duct IPMN (BD-IPMN), as reported in a recent consensus statement.21,22
CURRENT SCREENING STUDIES
Biochemical Screening
CA 19-9 is a Lewis antigen that is not expressed in 5–10% of the population, implying that at least 5–10% of the population cannot be screened using this marker.23 CA 19-9 also becomes elevated in patients with benign pancreatobiliary disorders, chronic pancreatitis, and other causes of cholestasis.23 Although CA 19-9 is one of the best single markers in terms of sensitivity and specificity, the elevated values in benign conditions make CA 19-9 less useful as a screening marker. This has been exemplified in several of the screening studies conducted in Japan and Korea;24,25 in these studies, which screened >80,000 subjects, the results were dismal for CA 19-9 as the single marker of PC. Although the sensitivity and specificity were excellent at 100% and 98%, respectively, the positive predictive value was poor at 0.03–0.09%.24,25 These values make CA 19-9 excellent for ruling out, but not for ruling in, PDAC. If one were to act on a positive CA 19-9 at the 37 U/mL threshold, significant unnecessary procedures would be performed, since only eight cancers were detected in the screened patients.24,25 The conclusions drawn from these large-scale screening studies are that CA 19-9 screening of asymptomatic patients is ineffective, but it is an effective marker for symptomatic patients.
In high-risk patients, the use of CA 19-9 to screen is more effective; however, not all lesions identified are adenocarcinomas.26 Using a combination of CA 19-9 and endoscopic ultrasound (EUS)-guided fine needle aspiration (FNS), Zubarik et al.26 evaluated 546 high-risk individuals, of whom 27 had elevated CA 19-9. Follow-up EUS identified five individuals with pancreatic lesions, of which only one lesion was PDAC. In this small study, the positive predictive value was 3.7%, significantly higher than screening asymptomatic patients.
By adding additional markers to CA 19-9, it may be possible to improve the effectiveness of biochemical screening of asymptomatic patients.27 In a longitudinal study of the prostate, lung, colon, and ovarian screening cohort, multiple markers were evaluated for detecting PDAC at >1 and <1 year prior to actual diagnosis. This produced a panel consisting of CA 19-9, carcinoembryonic antigen, and Cyfra 21-1. This combination produced a higher sensitivity of 30% compared to 17% for CA 19-9 alone, at a specificity of 95%. These studies conclude that a stand alone screening cohort for identifying a PDAC biomarker may be difficult to accrue but may be more easily recruited as part of a larger screening study for multiple cancers.
In a recent publication, Fric et al.28 proposed a new algorithm for screening the sporadic PDAC in the general population based on the endocrine function of the pancreas, suggesting that glucose intolerance is a sign of early PDAC. According to their algorithm, the following patients aged >50 years should be screened for PDAC: patients with new-onset diabetes with decreasing body weight, low body mass, or unstable diabetes requiring insulin therapy, or long-term diabetic patients with new-onset resistance to prior treatment and weight loss.28 These are logical applications based on the physiological function of the pancreas, but require further clinical validation.
Imaging Screening
Imaging studies have not been used to screen the general asymptomatic population due to the significant cost incurred with these studies.1 To improve the screening yield, screening research has focussed on high-risk individuals. The definition of high-risk population has been stated by the International Cancer of the Pancreas Screening Consortium (CAPS).29 These patients include those with at least one first-degree relative affected by PDAC at age <50 years and patients with genetic mutations and cancer syndromes.26,30
When screening these patients, the most common screening technique has been either EUS or magnetic resonance cholangiopancreatography. EUS is most sensitive at detecting pancreatic lesions and can detect lesions <1 cm in size, which were not detectable by computed tomography (CT) or magnetic resonance imaging (MRI).29 The reported rates of detection were 43% for EUS, 33% for MRI, and 11% for CT.31,32 EUS also has the advantage of being able to sample the lesion at the time of scanning; therefore, based on these studies, EUS is currently the recommended technique for screening high-risk individuals.
In high-risk patients, the incidence of abnormalities on imaging is around 30–45% when MRI is involved.26,31,32 Magnetic resonance cholangiopancreatography, in addition to having high sensitivity, has positive and negative predictive values that are helpful in tumour staging and determining respectability. On the other hand, MRI provides excellent contrast resolution and detects small tumours on gadolinium-enhanced fat-suppressed images. In these studies, the identified lesions are predominantly cystic-related to either cystic or mucinous neoplasms (ranging between 50% and 90% of the abnormal findings) and PDAC was identified in between 0% and 4% of the screened patients. Mucinous lesions are present in 2–30% of the screened patients and dysplasia and early malignancy were frequently present in the mucinous lesions. Thus, even in high-risk patients, PDAC is present in a low percentage of patients, although the relative risk is significantly higher than the general population. Identifying the mucinous lesions early allows for early surgery to prevent eventual development of PDAC, and this may be the most significant benefit of screening the high-risk population. Table 2 outlines the sensitivities and specificities of different imaging modalities in the detection of PDAC.
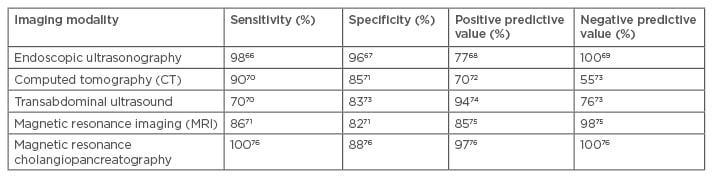
Table 2: Sensitivity, specificity, positive predictive value, and negative predictive value of various imaging modalities used in the diagnosis of pancreatic ductal adenocarcinoma.
Molecular and Genomic Screening
Reliable molecular markers can help in determining the nature of pancreatic lesions identified by imaging, especially during the screening of high-risk individuals. Due to the size and non-invasive nature, microscopic precancerous lesions (PanIN and IPMN) are likely to be insensitive to detection using serum markers.
Circulating Tumour Cells and DNA
Pancreatic juice contains high concentrations of DNA and other molecules released from PC; thus, molecular alterations can be readily detected as opposed to other specimens such as blood or stool.33 The fluid can be collected during routine upper gastrointestinal endoscopy after secretin infusion, making it a potential specimen to analyse markers in patients with diffuse abnormalities of the pancreas by imaging; on the other hand, FNA is best used to sample focal lesions detected by imaging. A study showed that circulating tumour DNA (ctDNA) was detectable in 48% of patients with PDAC, making it a potential marker to be used in conjunction with other panels.34 Other than improvement in sensitivity, it is important to standardise pre-analytical processes for ctDNA analysis, such as blood sample acquisition, plasma separation, ctDNA extraction, and quantification; ctDNA analysis will need further clinical validation in larger prospective studies in patients with early stage disease.
Mesothelin
Mesothelin is a glycoprotein that acts as a tumour antigen and can be detected in FNA from suspected pancreatic lesions.35 Mesothelin-specific T cells can be induced in patients with PDAC, making it a potential target for immune-based interventions.36 However, it still remains to be determined if the gene products could serve as diagnostic serum markers of PC for screening.
Macrophage Inhibitory Cytokine-1 and Other Protein Markers
Markers are elevated in the serum of patients with PDAC.37 Macrophage inhibitory cytokine-1 (MIC-1) is a more sensitive marker of PDAC than CA 19-937 and is a distant member of the transforming growth factor-beta superfamily, originally identified in the setting of macrophage activation.38 MIC-1 is overexpressed in several cancer types, including pancreas, colon, prostate, breast, and gastric cancers,39-41 and has demonstrated in vivo and in vitro effects on tumour growth and/or apoptosis.42 In a recent study37 of 50 patients with resectable PC, 50 with chronic pancreatitis, and 50 healthy, age and sex-matched controls, MIC-1 performed significantly better than CA 19-9 at differentiating patients with PC from healthy controls (area under the curve: 0.99 versus 0.78; p=0.003). A total of 90% of the patients with resectable PC had MIC-1 levels >2 standard deviations above age-matched controls, whereas only 62% had elevated CA 19-9, and, unlike CA 19-9, MIC-1 elevations were independent of tumour, node and metastasis (TNM)stage; 6 of 7 patients with T1 or T2 cancers had elevated MIC-1, whereas only 2 of 7 had elevated CA 19-9. In contrast, MIC-1 was no better than CA 19-9 in distinguishing patients with chronic pancreatitis from those with PC,37 an important feature since this distinction is difficult to make using clinical and radiographic criteria. These results suggest that serum MIC-1 could be helpful in the early detection of PDAC in high-risk cohorts as a part of their pancreatic screening protocols.
In other studies, serum markers like matrix metalloproteinase 7 and adenosine deaminase successfully distinguished pancreatic adenocarcinoma from chronic pancreatitis, but showed no improvement in accuracy compared to CA 19-9 alone.37,43 A new monoclonal antibody to mucin 1, a membrane-associated glycoprotein that is overexpressed in multiple cancers including PDAC and from which the CA 19-9 antigen is derived, satisfied both criteria to differentiate PDAC from normal state and chronic pancreatitis. However, its sensitivity and specificity were 77% and 95%, respectively, remaining below desirable accuracy levels.44
Mutant KRAS
KRAS mutations are present in approximately 90% of PDAC cases, limited to one codon, and can be readily detected using molecular assays.33 However, KRAS mutations are not specific for PC and can also be present in smokers and patients with chronic pancreatitis and PanIN.45,46 It is possible that quantifying mutant KRAS levels in the blood and pancreatic juice could improve the diagnostic utility of mutant KRAS and potentially be used as a screening tool.47
TP53
TP53 mutations occur relatively late in the neoplastic process towards invasive PC and are found in approximately 70% of invasive PDAC cases.48 A few nucleotide hot spots of the TP53 gene mutation are known, but mutations occur throughout the gene.49 Thus, the detection of TP53 mutations in pancreatic juice has the potential to be a useful diagnostic and screening strategy, particularly if improvements in mutation detection technology can enable accurate detection of such mutations at low concentrations.
Methylated Genes
Numerous genes undergo aberrant methylation during the neoplastic process and are rarely detected in non-neoplastic pancreatic tissues. These genes include p16, ppENK, Cyclin D2, SOCS1, SPARC, and TSLC1 and can be detected via methylation-specific polymerase chain reactions, making them potentially attractive for early detection protocols.33
Other markers like SPAN-1, CA-50, DUPAN-2, elastase-1, tissue polypeptide antigen, and tissue polypeptide-specific antigen have been studied but have not performed well.50 In addition, microscopic precancerous lesions like PanIN and IPMN are insensitive to detection using these serum markers due to their small size and non-invasive nature. Larger panels, with appropriate molecular marker combinations, can improve accuracy in pancreatic adenocarcinoma diagnosis.51 Table 3 outlines the sensitivities, specificities, and other statistical significances of these novel molecular and genetic markers.
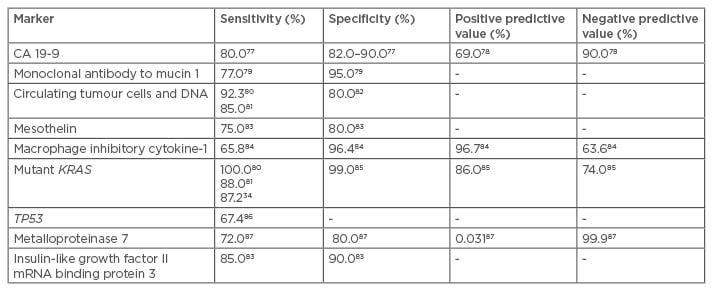
Table 3: Sensitivity, specificity, positive predictive value, and negative predictive value of the emerging biochemical, molecular, and genetic markers.
DIRECTIONS AND CHALLENGES
Primary PC contains a mix of distinct subclones, each containing hundreds of millions of cells that are present within the primary tumour years before the metastases become clinically evident.52 There can be an interval of >1 decade for genetic progression of PC from initiation to the metastatic stage, suggesting that a window of opportunity for early detection exists.53 Currently, several screening modalities have been implemented in high-risk cohorts to increase the pretest probability of testing.
One such computer-based risk assessment tool, PancPRO, assigns a quantitative risk score to any member of families with familial PC.54 An Italian PDAC registry study evaluating this model demonstrated that having a PancPRO risk score >10 is one of the major criteria for enrolment in a screening programme.55 High-risk patients are screened using a multimodality screening combination of a CT and an EUS; an abnormal EUS will be followed by an EUS-FNA and an endoscopic retrograde cholangiopancreatography.56
The objective of developing a robust screening programme is to provide early resection for the aforementioned precancerous lesions. Based on the size and internal features of the lesions, follow-up imaging with MRI should be performed every 1–2 years for lesions <3 cm in size and those without intracystic solid nodules. These benign-appearing lesions need to be monitored for ≥5 years.22 While resection is recommended for main duct IPMN and MCN, high-risk stigmata and worrisome features like enhanced solid component, MPD size of >10 mm, cyst size of >3 cm, thickened enhanced cyst walls, non-enhanced mural nodules, MPD size of 5–9 mm, abrupt change in the MPD calibre with distal pancreatic atrophy, and lymphadenopathy have been defined to stratify the risk of malignancy in BD-IPMN to consider resection versus increased frequency of surveillance.22 Some authors advocate continuation of surveillance every 6 months in view of the relatively high incidence of PDAC in patients with BD-IPMN; on the other hand, PanIN can only be identified reliably after surgical resection. Thus, suspicion of pancreatic lesions on imaging screening in these high-risk cohorts should be followed by a biomarker panel to suggest high-risk PanIN leading to resection, versus low-risk PanIN, which can be monitored by close surveillance.
Several issues currently limit standardisation of surveillance. One obstacle to surveillance is the lack of knowledge regarding the natural history of premalignant lesions and the outcome of these lesions in high-risk patients. In several reports of PDAC cases, the volume doubling time of PDAC once visible by a CT scan ranged from 20–1,351 days;57 this could mean a potential surveillance frequency that ranges from 3–12 months. Another issue concernings lead time bias, when earlier detection of tumours via screening may seem to result in longer survival than those identified by clinical symptoms, although the natural history of the tumour may not have been altered. We also face challenges of tumours with aggressive biology where recurrence or metastases are seen even after resection.
CONCLUSION
PC is a deadly disease even though it has a low incidence in the general population. The fact that it can take more than a decade to progress from initiation to the metastatic disease provides us with a critical window of opportunity for early detection. Although we have made some clinically meaningful progress in screening the high-risk cohorts, non-selective population-based screening is certainly not ready for widespread use. Currently, there is no consensus on the most appropriate screening protocol for early PC; however, newer techniques using molecular and genomic modalities are promising. These tests, if validated, can provide an accurate and reliable modality to enable early diagnosis in patients that are asymptomatic. In addition, this will reduce PC-specific mortality and could prevent PC from becoming the second leading cause of cancer-related death in 2030.








