Abstract
Advanced urothelial cancer (aUC) is invariably lethal and standard of care, platinum-based chemotherapy has changed little over the past 25 years. However, the past 5 years have been transformational with the advent of immunotherapies and targeted therapies. In this review, the authors focus on the therapies that are showing the greatest promise and have changed, or will imminently impact, the treatment landscape of aUC. Checkpoint inhibition is showing deep and durable responses in some patients and trial activity is concentrated on identifying the most suitable position within the treatment paradigm along with the most appropriate patients and therapeutic combinations. Novel targeted therapies in aUC are gaining renewed interest with nectin-4 antibody drug conjugates and fibroblast growth factor receptor inhibitors, both receiving recent regulatory approvals. Bispecific antibodies, capable of binding to two targets at the same time, are also showing promise. This review discusses the preclinical data, the relevant past, and present clinical trials along with regulatory status to provide a concise overview of the current and impending treatment options for aUC.
INTRODUCTION
Urothelial cancer is the 9th most common cancer in the world and the 10th most common cancer in the UK.1More than 10,000 new urothelial cancer cases occur in the UK every year, with a quarter of the patients presenting with locally advanced or metastatic urothelial carcinoma. Incidence rates are highest in older people (aged 85–89 years) and despite current treatment options the 5-year survival remains at only around 10%.2
The treatment landscape of advanced urothelial cancer ([aUC]: locally advanced or metastatic urothelial carcinoma) is now rapidly evolving with a recent increase in the number of approvals by the European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA) (Figure 1).
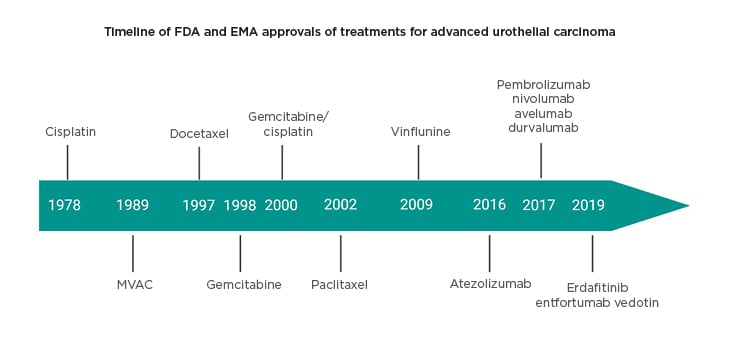
Figure 1: Timeline of approved agents in advanced urothelial cancer.
Agency (EMA) approvals of treatments for urothelial carcinoma.
EMA: European Medicines Agency; FDA: U.S. Food and Drug Administration.
This review summarises current treatment options for aUC, including cytotoxic chemotherapy and immune checkpoint blockade, with a focus on recent advances in targeted therapies and bispecific antibodies that are most likely to impact the management of aUC in the future.
CYTOTOXIC CHEMOTHERAPY
Platinum-based combination chemotherapy is currently the global first-line treatment for metastatic urothelial carcinoma.3 Combinations in use include MVAC (methotrexate, vinblastine, doxorubicin, cisplatin), gemcitabine and cisplatin, and gemcitabine and carboplatin. Response rates have been reported at around 30–40%. Second-line chemotherapy agents such as taxanes, vinflunine, ifosfamide, and oxaliplatin have only demonstrated modest benefits.4 For example, vinflunine, a microtubule inhibitor, led to only a 1.5 month improvement in progression-free survival (PFS) (median PFS: 3.0 versus 1.5; hazard ratio [HR]: 0.68; 95% confidence interval [CI]: 0.54–0.86; p=0.001) and 2.3 months improvement in overall survival (OS) (median OS: 6.9 versus 4.3 months; HR: 0.78; 95% CI: 0.61–0.99; p=0.04).5 Immune checkpoint inhibitors (CPI) are now routinely used instead of cytotoxic chemotherapy in the second-line setting.
IMMUNOTHERAPY
The past 4 years has seen regulatory approval of five separate CPI (Figure 1) for the treatment of aUC. The authors have presented some of the stronger trial data to support second-line, first-line, and maintenance CPI; emerging data of combination checkpoint inhibition; and then focus on an interesting future advance, bispecific antibodies.
Immune Checkpoint Inhibitors
The strongest current evidence for the use of CPI in aUC comes from the KEYNOTE-045 study, which compared pembrolizumab (PD-1 inhibitor) with standard of care chemotherapy in patients who had previously progressed on platinum-based chemotherapy.6,7 The co-primary endpoints were OS and PFS. With a median follow of 27.7 months, there was a 2.8 month improvement in survival with pembrolizumab compared to chemotherapy (median OS: 10.1 versus 7.3 months; HR: 0.7; 95% CI: 0.57–0.85; p<0.001) and in responders (response rate: 21.1% versus 11.0%) the median duration of response was substantially longer with the CPI (not reached versus 4.4 months). IMvigor2118 was a Phase III study comparing atezolizumab (anti-PD-L1) to standard of care chemotherapy in patients who had previously progressed on platinum-based chemotherapy. Atezolizumab was also active in the second-line setting but failed to reach the primary endpoint of improved OS in PD-L1 positive patients, partly because of the statistical design and better-than-expected performance of the chemotherapy control arm. Both agents are approved by the EMA for use in second-line treatment.
First-line CPI was initially tested in patients with aUC who were cisplatin-ineligible (renal impairment, neuropathy, or poor Eastern Cooperative Oncology Group [ECOG] performance status). This was following objective response rates (ORR) of 29% in the KEYNOTE-052 trial and 23% in the IMvigor 210 trials, two Phase II trials testing pembrolizumab and atezolizumab, respectively, in this setting,9 which have led to the approval of these agents. The subsequent Phase III studies (KEYNOTE-361 and IMvigor130) compared chemotherapy with chemoimmunotherapy or immunotherapy alone in first-line metastatic disease. An interim analysis of these two studies suggested that CPI monotherapy may be less effective than chemotherapy in patients with low PD-L1 expression in the first-line setting,10 leading to an EMA restriction of CPI monotherapy to patients with high PD-L1 expression. The initial results of IMvigor130, after a median of 11.8 months, have been reported in abstract form showing that atezolizumab plus chemotherapy leads to a 1.9-month improvement in PFS, the co-primary endpoint, compared to chemotherapy alone (median PFS: 8.2 versus 6.3 months; HR: 0.82; 95% CI: 0.70–0.96; p=0.007). There was a 2.6 month numerically higher OS, the other co-primary endpoint, for atezolizumab plus chemotherapy (median OS: 16.0 versus 13.4 months; HR 0.83; 95% CI: 0.69–1.00, p=0.027) but did not meet the prespecified interim boundary for significance. Outcomes of longer follow-up of IMVigor130 for OS remain unknown, but whether this is adopted will be contingent on the balance between survival and toxicity.11
Given the short PFS after first-line therapy and a significant fall off in the number of patients receivingsecond-line therapy, attributable to a decline in fitness, maintenance CPI has been tested in patients that had at least stable disease following 6–8 cycles of first-line platinum-based chemotherapy. Maintenance pembrolizumab led to a 2.6-month improvement in predicted median PFS (8.2 versus 5.6 months; p=0.023) compared to placebo.12 Although not yet presented, Pfizer had announced following the planned interim analysis that the JAVELIN Bladder 100 study of avelumab maintenance versus standard of care met its co-primary endpoint prolonging OS in patients with PD-L1-positive tumours.13 Given that there are now two positive studies in this setting, maintenance CPI may become a new standard of care.
Combination Immunotherapy
Given that response rates to CPI monotherapy are only in the order of 20–25%, other approaches are required to progress these agents. CPI is being tested in combination with chemotherapy as discussed above, but also with other CPI or with targeted therapies. However, these combination approaches have so far been disappointing, as exemplified by the DANUBE14 and BISCAY15 studies. A recent press release announced that the DANUBE study, a randomised Phase III trial of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA4) versus chemotherapy in first-line metastatic urothelial cancer failed to meet either of its co-primary endpoints, OS or OS in patients with high PD-L1 expression.14 Two similar Phase III studies for first-line aUC are ongoing with the NILE study16 testing triplet therapy (durvalumab and tremilimumab and chemotherapy), and the Checkmate901 testing an alternative doublet CPI (ipilimumab and nivolumab)17 are still to report and help clarify whether CPI-CPI combinations or CPI-chemotherapy combinations are of benefit in aUC. In BISCAY,15 a biomarker-driven Phase II study that explored either durvalumab monotherapy or durvalumab in combination with poly (adenosine diphosphate ribose) polymerase inhibitors, fibroblast growth factor receptor (FGFR)3 inhibitors, or mTOR inhibitors as second-line therapy in aUC, no combination treatment met the prespecified efficacy target.
Bispecific Antibodies
Bispecific antibodies are capable of binding to two targets at the same time.18 These are early-stage molecules with most of the data derived from basic science and cell-line research, with some Phase I studies recruiting. One strategy of using a bispecific antibody is by targeting CD3 and a tumour antigen simultaneously. This can recruit and activate T cells for effective tumour clearance by bringing the T cells closer to the cancer and activating them via the CD3 receptor pathway. Another strategy is to simultaneously target immune coinhibitory and costimulatory receptors. These receptors can be upregulated on activated T cells, including regulatory T cells, in the tumour microenvironment, and therefore using bispecific antibodies may increase the localisation of the antibodies to the tumour and improve tumour-specific clearance and reduce immune-related adverse events.
Preclinical data has suggested that bispecific antibodies may be a viable therapeutic option in urothelial cancer. B7-H3 is highly expressed on urothelial cancer cells. A CD3 and B7-H3 bispecific antibody armed on T cells demonstrated increased cytotoxicity towards bladder cancer cells. Secretion of IFN-γ and TNF-α was increased compared to unarmed activated T cells.19,20 The same group demonstrated similar results with a bispecific antibody against CD3 and CD155 armed on activated T cells, again demonstrating their results on bladder cancer cell lines. MGD009 is a humanised anti-B7-H3 and anti-CD3 bispecific antibody that is being evaluated for safety in a multicentre, open-label, Phase I dose escalation and cohort expansion study21including patients with urothelial cancer. A Phase I study of MGD009 with an anti-PD1 antibody, MGA01222 is currently recruiting.
A CTLA-4 and OX40 bispecific antibody resulted in T-cell activation and regulatory T-cell depletion in vitro.23Using syngeneic mouse models of bladder cancer, injections of this antibody resulted in durable tumour clearance.
LY3415244 is a TIM-3 and PD-L1 bispecific antibody. It is hoped that intrinsic and acquired resistance to immune checkpoint inhibition can be overcome by targeting and inhibiting both these co-inhibitory receptors. J1C-MC-JZDA is a multicentre, nonrandomised, open-label, Phase Ia/Ib study assessing LY3415244. The Phase Ia study will recruit patients with any tumour type and the Phase Ib expansion cohorts will recruit patients who have previously received a PD-1 or PD-L1 inhibitor, in non-small cell lung cancer, urothelial cancer, and melanoma.24 Similarly, RO7121661 is an anti-PD-1/TIM-3 bispecific antibody that entered Phase I studies for treatment of patients with metastatic solid tumours.25 Bispecific antibodies targeting other immune co-inhibitory checkpoints are running in Phase I studies. MGD013 targets PD-1 and LAG-3 and is recruiting to Phase I.26
TARGETED THERAPIES
Multiple targeted agents, including small molecule inhibitors or antibodies, have been tested against vascular endothelial growth factors, epidermal growth factor receptors, mTOR, and the cell cycle. Unfortunately, none of these agents demonstrated sufficient activity or efficacy in trials to gain regulatory approval (Table 1).6,27-43 Recently, two targets, nectin-4 and FGFR, have demonstrated great promise and are discussed in more detail here.
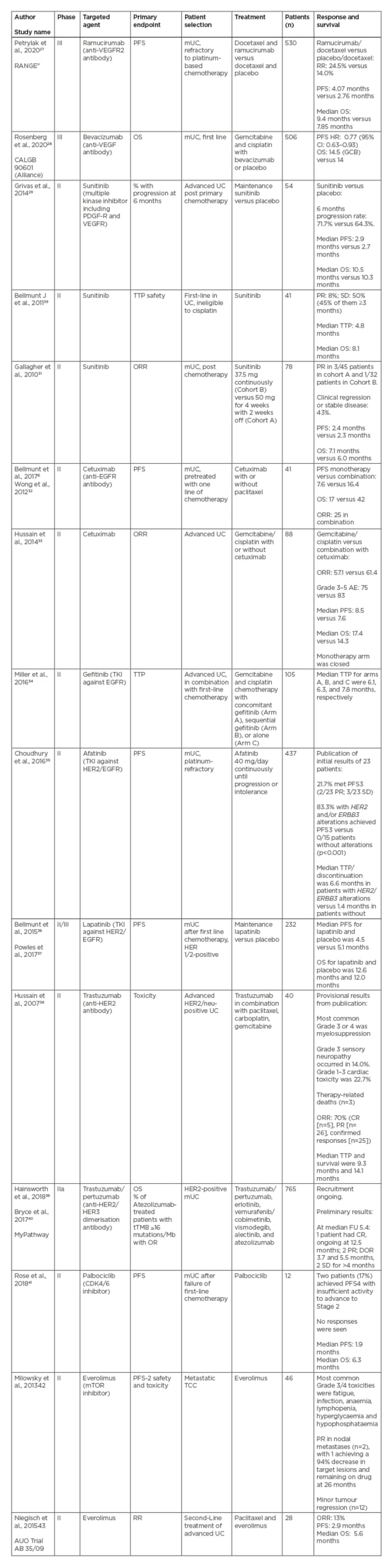
Table 1: Summary of clinical trials of targeted agents in advanced urothelial cancer that have not gained regulatory approval.6,27-43
AE: adverse event; CR: complete response; CPS: combined positive score; DOR: duration of response; EGFR: epidermal growth factor receptor; FGFR: fibroblast growth factor receptor; FU: follow-up; GCB: germinal centre B-cell; HR: hazard ratio; HER2: human epidermal growth factor receptor; mTOR: mammalian target of rapamycin; mUC: metastatic UC; OR: overall response; ORR: overall response rate; OS: overall survival; PDGF-R: platelet-derived growth factor receptor; PD-L: programmed death ligand; PFS: progression-free survival; PR: partial response; RR: response rate; SD: stable disease; TEAE: treatment-emergent adverse events; TKI: tyrosine kinase inhibitor; tTMB: tissue tumour mutational burden; TTP: time to progression; UC: urothelial cancer; VEGFR: vascular endothelial growth factor receptor; 95% CI: 95% confidence interval.
Nectin-4
Antibody–drug conjugates (ADC) enable the delivery of high concentrations of cytotoxic chemotherapy to tumour cells by enabling targeted delivery through conjugation with a monoclonal activity. This strategy has become a standard of care in some malignancies, for example TDM-1 in breast cancer.
Enfortumab vedotin (EV) is an ADC that binds to nectin-4, a transmembrane protein that regulates a number of cellular functions including angiogenesis, and is highly expressed in multiple tumours including urothelial, ovarian, lung, breast, and gastric.44-46 Upon binding to nectin-4, EV is internalised into the cell where cytoplasmic proteases cleave the linker between the nectin-4 antibody and the drug payload monomethyl auristatin E (MMAE) (vedotin is an MMAE and a protease-cleavable linker to an antibody),47 releasing its cytotoxic activity. More specifically, MMAE inhibits tubulin polymerisation, leading to mitotic arrest and downstream apoptotic cell death.
Given that nectin-4 is expressed by 97% of aUC,48,49 a global, Phase II, single-arm study of EV in patients with aUC who had previously been treated with platinum-based chemotherapy and anti-PD-1 or PD-L1 immune CPI was performed.48 Here, 125 patients were treated with EV that was administered intravenously on Days 1, 8, and 15 of a 4-week cycle. The primary endpoint was ORR using the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 criteria. Secondary endpoints included duration of response, PFS, OS, safety, and tolerability.
Nectin-4 expression was assessed by immunohistochemistry (using a modified H-score, a continuous weighted scale).50 The ORR was 42% (95% CI: 35.1–53.2%), with 12% complete responses, which is markedly higher than any other third-line treatment that has been tested in aUC. The median duration of response was 7.6 months (range: 0.95–11.30 months). The estimated median PFS was 5.8 months (95% CI: 4.9–7.5 months) and the estimated median OS was 11.7 months (95% CI: 9.1 months to not reached). As well as this prolonged survival compared to historical controls, EV was also well tolerated with the most common grade ≥3 toxicities being neutropenia (8%), anaemia (7%), and fatigue (6%). Treatment-related adverse events led to dose reductions in 32% of patients and discontinuation of treatment in only 12% of patients, with no treatment-related deaths. The most common toxicities of any grade were fatigue (50%), alopecia (49%), decreased appetite (44%), dysgeusia (40%), peripheral sensory neuropathy (40%), nausea (40%), diarrhoea (40%), and maculopapular rash (27%). Based on results from the EV-201 trial, the FDA granted accelerated approval to EV in December 2019. The global registration Phase III study of third-line EV compared to standard of care chemotherapies (EV-301) has recently completed data accrual and initial results are expected towards the end of 2020.51
Given this encouraging level of activity in the third-line setting, EV has also been tested in combination with pembrolizumab (anti-PD-1) as a first-line therapy in patients who were ineligible to receive platinum chemotherapy. Initial results were presented at ESMO 2019,52 and updated at GU ASCO 2020.53 The overall response rate was 73.3% with a complete response rate of 15.6%. At a median follow up of 10.4 months, 55% of the responders had an ongoing durable response. Treatment-related toxicities included fatigue (58%; 11% ≥G3), alopecia (53%), and peripheral sensory neuropathy (53%; 4% ≥G3). However, one patient in the study died as a result of multiple organ failure. The FDA granted breakthrough designation to the EV and pembrolizumab combination in February 2020. This combination demonstrates encouraging efficacy compared to previous drugs used in the first-line setting and, if replicated in Phase III studies, could lead to a paradigm shift in the treatment of aUC.52,53 The EV-302 Phase III study will evaluate the EV and pembrolizumab combination therapy versus standard of care gemcitabine and platinum in the first-line treatment setting for aUC, with primary outcome measures of PFS and OS.
Fibroblast Growth Factor Receptor
FGFR activation results in signal transduction via the downstream MAPK and PI3K pathways, which regulate tumour survival and growth.52 FGFR3 alterations are present in approximately one-fifth of patients with urothelial bladder cancer and in one-third of patients with upper tract urothelial carcinomas.54
Erdafitinib is a potent inhibitor of FGFR1–4 and a weaker inhibitor of VEGFR2. It has been the first targeted anticancer therapy to gain accelerated approval by the FDA55 for patients with aUC carcinoma with susceptible FGFR2 or FGFR3 mutations, based on the results of the BLC2001 study.56 Simultaneously, approval for the Therascreen® (Qiagen, Hilden, Germany) FGFR RGQ RT-PCR Kit was given as the companion diagnostic. This is a reverse-transcriptase-PCR assay that tests for specific FGFR3 mutations or FGFR2/3fusions using RNA extracted from formalin-fixed paraffin-embedded samples.
The BLC2001 study was an open-label Phase II study enrolling patients with aUC with prespecified FGFRalterations56 to treatment with oral erdafitinib. In total, 99 patients with specified FGFR3 gene mutations or FGFR2/3 gene fusions were recruited. Patients had to have progressed following treatment with one course of chemotherapy or within 12 months after neoadjuvant or adjuvant chemotherapy. The primary endpoint was ORR and secondary endpoints included duration of response, PFS, and OS.
Patients were initially randomised in a 1:1 ratio to either receive an intermittent regimen (10 mg per day, with daily administration for 7 days and off for 7 days) or a continuous regimen (6 mg per day). Subsequently, a planned interim analysis of safety and efficacy was performed in June and July 2016 and further enrolment to the intermittent-regimen group was halted. In August 2016, the study was converted to a single-group analysis following a protocol amendment to increase the starting dose to 8 mg per day in a continuous regimen.
The ORR was 40% (95% CI: 31–50), the median duration of response was 5.6 months (95% CI: 4.2–7.2). The median PFS was 5.5 months (95% CI: 4.2–6.0) and median OS was 13.8 months (95% CI: 9.8–not reached). Patients with FGFR3 mutations were noted to have a better ORR (49%) compared to those with FGFR2/3fusions (16%).
In terms of safety, 46% of patients experienced a treatment-related adverse event at Grade 3 or higher. The most commonly reported toxicities that were Grade 3 or higher were hyponatraemia (11%), stomatitis (10%), and fatigue (7%) and 13 patients had treatment discontinuation. This was because of detachment of the retinal pigment epithelium, hand-foot syndrome, dry mouth, and skin or nail events. Furthermore, 55 patients required a dose reduction, which was commonly a result of stomatitis (16 patients) and hyperphosphataemia (9 patients). Common adverse events included hyperphosphataemia (77% all grade), stomatitis (58% all grade), diarrhoea (51% all grade), and dry mouth (56% all grade). Hand-foot syndrome was at 23% any grade. Hyperphosphataemia, a class effect of FGFR inhibition,57 which is thought to be secondary to inhibition of FGF23 signalling,58 could be a useful pharmacodynamic biomarker. A randomised Phase III study (THOR)59is now investigating the benefit of erdafitinib compared with chemotherapy or pembrolizumab, with a primary outcome of OS. Patients who have progressed on or after one or two prior treatments, at least one of which includes an anti-PD-1/PD-L1 agent (Cohort 1) or one prior treatment not containing an anti-PD-1/PD-L1 agent (Cohort 2).
Other FGFR inhibitors include infigratinib, rogaratinib, pemigatinib, and Debio 1347 (Debiopharm, Lausanne, Switzerland). Infigratinib (BGJ398) is a potent and selective FGFR1–3 inhibitor.60 An exploratory analysis of Phase II data61 demonstrated a difference in ORR in upper urinary tract urothelial carcinoma (50%) compared to lower urinary tract urothelial carcinoma (22%). The difference in ORR could be because of differences in genomic alterations between the two patient groups. A higher frequency of FGFR3-TACC3fusions (12.5% versus 5.8%) and FGFR3 R248C mutations (50% versus 11.5%), and a lower frequency of FGFR3 S249C mutations (25% versus 59.6%) was found when comparing upper with lower urinary tract urothelial carcinoma.
Rogaratinib (BAY1163877) is a potent and selective inhibitor of FGFR 1–4.62 Results from the Phase I study were reported in 2016.63 A Phase III trial64 comparing rogaratinib against chemotherapy in metastatic urothelial carcinoma who have received prior platinum-based chemotherapy is currently active but not recruiting in January 2020.
The interim results of the FIGHT-20165 study, a Phase II, open-label, multicentre study of pemigatinib (INCB054828), was reported in 2018. Patients had to have previously progressed on one or more treatments and had FGFR3 mutations or fusions (Cohort A) or other FGF/FGFR gene alterations (Cohort B). 64 patients were in Cohort A and with an ORR of 25% (95% CI: 14–40%). There were no responses determined by RECIST 1.1 in Cohort B. FIGHT-205,66 looking at pemigatinib plus pembrolizumab versus pemigatinib alone versus standard of care for participants with metastatic or unresectable urothelial carcinoma who are not eligible to receive cisplatin, are harbouring FGFR3 mutation or rearrangement, and who have not received prior treatment, is currently recruiting.
Debio 1347 is a selective inhibitor of FGFR 1–3. The FUZE trial is an ongoing Phase II basket trial in FGFRfusion-positive advanced solid tumours irrespective of tumour histology, enrolling patients with aUC with at least one prior treatment line.67 The primary endpoint is ORR.
Targeting FGFR3 alone in pretreated patients has not demonstrated similar levels of ORR compared to the multitargeted FGFR inhibitors. A Phase II study of dovitinib, a multitargeted tyrosine kinase inhibitor with activity against FGFR3 looked at 44 aUC patients who progressed after at least one chemotherapy regimen.68 Patients were classified as FGFR3 mutant or wild type. The study was not taken further because of a lack of ORR (0%; 95% CI: 0.0–26.5).
Small molecule tyrosine kinase inhibitors are not the only strategy to target the FGFR pathway in urothelial carcinoma. Vofatamab (B-701) is a fully human monoclonal antibody against FGFR3 that blocks activation of the wild type and genetically activated receptor. FIERCE-21 is a Phase Ib/2 study designed to evaluate vofatamab monotherapy or in combination with docetaxel69 in metastatic urothelial carcinoma with at least one treatment failure. The follow-up is immature at this time; however, data presented at ASCO GU 2019 showed that five out of 21 patients have had a partial response in the vofatamab combination arm compared to one out of 21 patients in the monotherapy arm.
CONCLUSION
This review discussed strategies that allow better targeting of aUC. ADC in the form of EV demonstrate good response rates in pretreated metastatic disease, and early results in the first-line setting are encouraging. There are now actionable genomic alterations in the form of FGFR inhibitors that can lead to better outcomes in selected groups of patients. Bispecific antibodies may allow urothelial cancer cells to be targeted specifically and overcome mechanisms of resistance to immune CPI.
There have been advances in developing targeted and personalised therapies in metastatic urothelial carcinoma. Further discussed here were three different targeted agents demonstrating promise in both clinical trials and preclinical research (Figure 2).
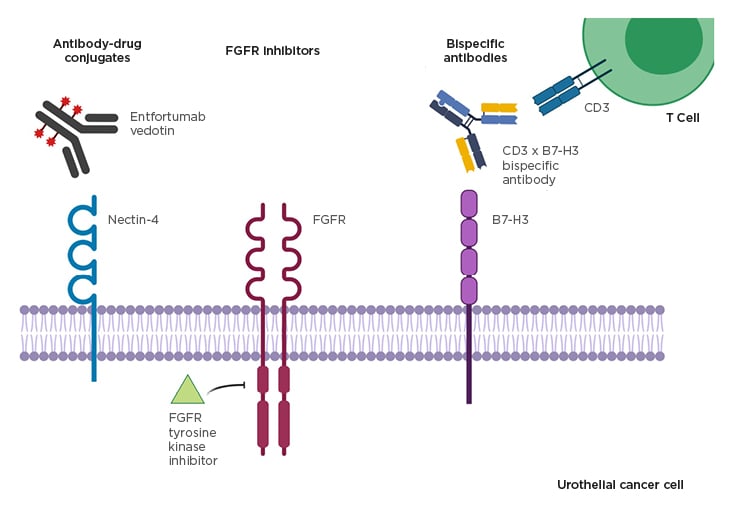
Figure 2: Promising targeted treatment strategies in advanced urothelial cancer.
FGFR: fibroblast growth factor receptor.
The development of targeted agents in aUC has positive implications for patients’ outcomes and treatment options. The challenge remains in optimising patient selection, sequencing of treatment, and whether combination strategies can lead to better outcomes. This represents a paradigm shift in the treatment of metastatic urothelial cancer, where previously treatment options were limited to cytotoxic chemotherapy.







