Meeting Summary
During this symposium, held at the 10th Congress of the European Academy of Neurology (EAN), speakers highlighted that chronic insomnia disorder (CID) is under-recognised and under-treated. Comorbid disorders associated with CID include psychiatric conditions, neurological disorders, and cardiovascular disease. Untreated, CID can negatively impact mental, physical, and occupational health. Consequently, the presence of CID should be evaluated and actively treated independent of comorbidities.
The concept of CID is characterised by a perpetuating cycle of hyperarousal. It is proposed that dual orexin receptor antagonists (DORAs) reduce hyperarousal and restore sleep–wake balance via antagonism of orexin 1 and orexin 2 receptors.
The European Insomnia Guidelines 2023 recommend cognitive behavioural therapy for insomnia (CBTi) as first-line treatment in adults. CBTi can be administered in-person or digitally. However, CBTi is not always available, can be costly in terms of time and resources, and not all individuals respond to therapy. Where CBTi is not effective or practical, the guidelines recommend short-term therapy (≤4 weeks) with benzodiazepines, benzodiazepine receptor agonists, the DORA daridorexant, or low-dose sedating antidepressants. DORAs can be used for >3 months in some cases, and prolonged-release melatonin for up to 3 months in individuals aged ≥55 years.
In Phase III trials, daridorexant reduced electroencephalography (EEG) features associated with hyperarousal in individuals with CID, reduced cumulative night-time waking, particularly time spent in long wake bouts, and improved daytime functioning. Real-world evidence showed that daridorexant improved sleep parameters in individuals with CID, including those with and without neurologic and psychiatric comorbidities.
Introduction
Chronic insomnia disorder is defined as difficulty falling asleep, staying asleep, and/or early-morning awakening experienced at least three times per week for at least 3 months, with impairment in daily functioning.1 Chronic insomnia disorder is the most prevalent sleep disorder worldwide, with 6–15% of adults globally meeting diagnostic criteria.1,2
Anna Heidbreder, Kepler University Hospital, Linz, Austria, explained that insomnia prevalence is not equal across the population: it increases with age,3 and is increased in specific population groups, including females and those with comorbid neurological conditions (e.g., Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, and restless legs syndrome).4-7 Furthermore, there is an association between chronic insomnia disorder and other major comorbidities, including cardiovascular disease, Type 2 diabetes, and mental health disorders (e.g., depression, anxiety, post-traumatic stress disorder).6,8,9
Disease Burden of Chronic Insomnia Disorder
Heidbreder emphasised that insomnia is a “24-hour disease,” as in addition to nighttime symptoms (difficulty initiating or maintaining sleep and/or early-morning awakening despite adequate opportunity for sleep), chronic insomnia disorder also impacts daily living. Indeed, insomnia can lead to difficulties with social, occupational, cognitive, academic, and behavioural functioning.3,10 Chronic insomnia can lead to difficulties with social, occupational, cognitive, academic and behavioural functioning, negatively impacting mental, physical and occupational health, and decreasing health-related quality of life (HRQoL).1,11 Additionally, there are implications for public safety, with an increased risk of road- and work-related accidents in individuals who have insomnia versus those without.1,12
Claudio Liguori, University Hospital of Tor Vergata, and Department of Systems Medicine, University of Rome Tor Vergata, Italy, highlighted that chronic insomnia disorder can exacerbate, or contribute to, comorbid conditions. For example, sleep alteration may be considered as a marker of early-stage Alzheimer’s disease.13 Furthermore, insomnia is highly prevalent in individuals with neurological disease (7.3% overall prevalence for any neurological condition), with particularly high prevalence in vascular dementia (67%), headache (53–61%), Parkinson’s disease (54–60%), epilepsy (34–58%), obstructive sleep apnoea (39–58%), mild cognitive impairment (44%), Alzheimer’s disease (25–35%), restless legs syndrome (17–26%), and multiple sclerosis (23%).6,7,14,15
Liguori stressed that, regardless of comorbidities, chronic insomnia disorder is recognised as a distinct condition, requiring specific and adequate management.16 As discussed later, recent real-world studies have investigated the impact of pharmacological treatment for chronic insomnia disorder in individuals with comorbid conditions, including obstructive sleep apnoea and psychiatric disorders.
Sleep Architecture in Chronic Insomnia Disorder
Yves Dauvilliers, Université de Montpellier, France, explained that sleep architecture varies across individuals with chronic insomnia disorder.16 Compared with a ‘good sleeper’, people with chronic insomnia disorder may show a high number of arousals and fragmented rapid eye movement (REM) sleep, or overall shortened sleep duration on EEG.16 Common to both of these profiles is a perpetuating cycle of hyperarousal.17,18
The Role Played by Orexin in Sleep–Wake Balance and Hyperarousal
Dauvilliers explained that, in the absence of insomnia, sleep–wake balance is mutually inhibitory and anchored by the neuropeptide, orexin.19,20 On the contrary, in individuals with chronic insomnia disorder, it is thought that a dysregulation of orexin activity occurs. Therefore, antagonism of orexin 1 and orexin 2 receptors by DORAs is needed to reduce orexin activity and effectively ‘switch off’ hyperarousal, restoring the sleep–wake balance (Figure 1).17,18
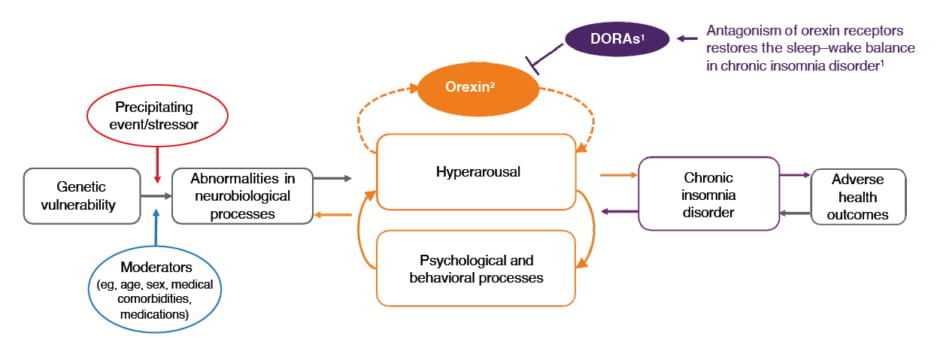
Figure 1: Dual orexin receptor antagonists act on the orexin system to ‘switch off’ hyperarousal in insomnia.17,18
Figure adapted from Levenson JC et al.17
DORAs: dual orexin receptor antagonists.
Evidence for Hyperarousal as an Underlying Cause of Insomnia
Hyperarousal has been investigated as an underlying cause of insomnia using multiple methodologies. Dauvilliers presented results from a post hoc analysis including pooled data from clinical trials and polysomnography (PSG) studies. EEG features associated with hyperarousal were increased in people with chronic insomnia disorder versus those without. Specifically, during wake and sleep Stage 1 (N1), individuals with insomnia had increased relative alpha and theta power, but reduced relative delta power, compared with those without insomnia. In addition, during N2, relative delta power decreased in those with insomnia versus those without insomnia.21
Data from PET further support a role for hyperarousal in chronic insomnia disorder. In a study assessing regional cerebral glucose metabolism, higher metabolic activity in wake-promoting brain regions, including the anterior cingulate cortex, hypothalamus, and ascending reticular activating system were detected during wake-to-non-REM (NREM) sleep transition in those with insomnia versus controls.22,23 This failure of arousal mechanisms to decline during wake-to-sleep transition may translate into an inability to fall asleep. Additionally, individuals with insomnia had reduced metabolic activity in wake-promoting brain regions while awake compared with controls, potentially explaining the fatigue experienced during the day by individuals with chronic insomnia disorder.22 It is postulated that this daytime impairment may reflect decreased activity in the prefrontal cortex resulting from inefficient sleep.
The European Insomnia Guidelines 2023
Diego García-Borreguero, Sleep Research Institute, Madrid, Spain, presented an overview of the recently updated European Insomnia Guidelines 2023.24 Within the guidelines, recommendations for diagnosis and treatment are graded according to an alphabetical system, where (A) represents a ‘Very Strong’ recommendation, and (B) represents a ‘Strong’ recommendation.
Diagnostic Management Guideline Recommendations
Diagnosis of insomnia, with or without comorbidities, involves several stages, including:24
- general clinical history and examination (A), which can include consideration of former and present disorders; personality factors, work, and partnership situation; substance use; physical examination and laboratory testing; and information from a bed partner/caregiver;
- sleep history (A), which can include sleep complaints and daytime functioning; work/time circadian factors; sleep–wake patterns, including use of a sleep diary; information from a bed partner/caregiver;
- actigraphy, if suspicion of irregular sleep–wake schedules or circadian rhythm sleep–wake disorders, or leg movement during sleep (A), or to quantify rest activity (A); and
- PSG, if suspicion of other sleep disorders or comorbid sleep disorders (A), treatment-resistant insomnia (A), or suspicion of large discrepancy between subjective versus objective measures of sleep (B).
García-Borreguero stressed that quantitative criteria related to sleep-onset latency, sleep duration, or the frequency of nocturnal awakenings do not have to be fulfilled in order to diagnose chronic insomnia disorder, which mainly relies upon symptoms described by the patient.
Treatment Guideline Recommendations
García-Borreguero reiterated that the European Insomnia Guidelines 2023 state that insomnia disorders should always be actively treated (A).24 Additionally, in the presence of comorbid conditions, clinical judgement should be used to decide whether insomnia or the comorbid condition should be treated first, or whether both should be treated at the same time (A).24
CBTi is recommended as first-line therapy for chronic insomnia disorder, administered either in person or digitally (A).24 CBTi can be effective in adults of all ages, with or without comorbidities, when programmes are completed and recommendations are followed. However, CBTi may not be available or adequate for all people with insomnia. For example, there may be instances where insufficient numbers of CBTi providers lead to unacceptable delays in treatment initiation.16,25 When CBTi is not sufficiently effective or available, a pharmacological intervention can be offered or combined with CBTi.24
Pharmacological treatments for insomnia differ in their mechanisms of action (MoA), efficacy, and tolerability,26 and include:
- DORAs
- MoA: blockade of orexin 1 and orexin 2 receptors27
- benzodiazepines and benzodiazepine receptor agonists
- MoA: allosteric modulators of GABAA receptor subtypes, increase GABA binding28
- low-dose sedating antidepressants (off-label use)
- MoA: antihistaminergic effect, inhibition of serotonin, and norepinephrine reuptake29
- prolonged-release melatonin
- MoA: agonist of melatonin type 1 and type 2 receptors, mimicking the normal period of nocturnal melatonin secretion.30
The clinical treatment algorithm classifies treatments according to the recommended duration of therapy (Table 1).24 Benzodiazepines (A), benzodiazepine receptor agonists (A), and low-dose sedating antidepressants (B) can be used for the short-term treatment of insomnia (≤4 weeks).24 Longer-term treatment with these substances may be initiated in some cases, considering advantages and disadvantages (B).24 DORAs can be used for short-term treatment (≤12 weeks) (A) and periods of up to 3 months or longer in some cases (A), whereas prolonged-release melatonin is recommended for up to 3 months only in individuals aged >55 years (B).24
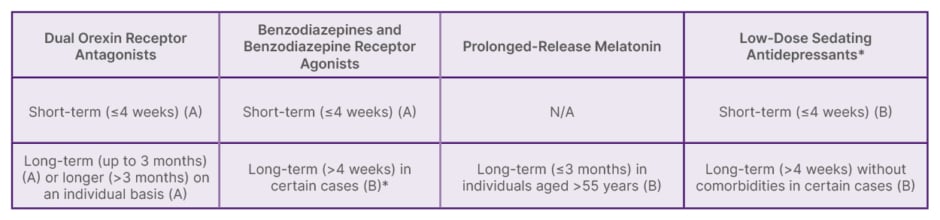
Table 1: European Insomnia Guidelines 2023: recommended pharmacological treatments (available in Europe) if cognitive behavioural therapy for insomnia is not effective.24
*Off-label use
(A): ‘Very Strong’ recommendation; (B): ‘Strong’ recommendation. N/A: not applicable.
Light therapy and exercise interventions may be used as adjunct therapies to CBTi (B).24 Antihistaminergic drugs, antipsychotics, fast-release melatonin, ramelteon, and phytotherapeutics are not recommended for insomnia treatment (A).24
The Dual Orexin Receptor Antagonist, Daridorexant, in the Treatment of Chronic Insomnia Disorder
Daridorexant is approved for the treatment of insomnia in adults in the USA, Europe, the UK, Switzerland, and Canada.31-35 Clinical trials showed that daridorexant improved subjective and objective sleep measures, as well as daytime functioning f or up to 12 months.36-38
Results from Phase III Clinical Studies
Dauvilliers presented the study designs for two pivotal Phase III studies of daridorexant (Study 301 and Study 302) and the optional study extension.36 Studies 301 and 302 were multicentre, randomised, double-blind, placebo-controlled Phase III trials that investigated different doses of daridorexant (50 mg or 25 mg in Study 301, and 25 mg or 10 mg in Study 302). In each study, participants were randomised to oral daridorexant (low dose or high dose) or placebo every evening for 3 months. Participants completed a daily e-diary assessment of sleep and daytime functioning (sleepiness and fatigue, mood, alertness, and cognition). The co-primary endpoints were change from baseline in wake time after sleep onset (WASO) and latency to persistent sleep (LPS), measured by PSG, at Month 1 and Month 3. The secondary endpoints were change from baseline in self-reported total sleep time (sTST) and sleepiness domain score of the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ©) at Month 1 and Month 3.
Dauvilliers presented results for daridorexant 50 mg (Study 301), which is the recommended dose approved by the European Medicines Agency (EMA).
In Study 301, daridorexant 50 mg significantly improved both sleep and daytime functioning.36 The WASO change from baseline to Month 3 with daridorexant 50 mg was -29.4 minutes (95% CI: -33.4, -25.4), which was significant versus placebo (p<0.0001).36 Daridorexant 50 mg also improved LPS at Month 3 (change from baseline to Month 3 was -34.8 minutes [95% CI: -38.1, -31.5]; p<0.0001 versus placebo).36
Regarding the secondary endpoints, change from baseline to Month 3 in sTST was +57.7 minutes (95% CI: 51.2, 64.2) in the daridorexant 50 mg group (p<0.0001 versus placebo), representing almost 1 hour increased sleep time.36 Change from baseline to Month 3 in the IDSIQ© sleepiness domain was also significantly improved with daridorexant 50 mg versus placebo (p=0.0002).36 Additionally, daridorexant 50 mg significantly improved IDSIQ© mood and alert/cognition domains (p≤0.0005 versus placebo at Month 3).36
Several post hoc analyses have been reported for Study 301. The finding from one post hoc analysis showed that daridorexant reduced EEG features associated with hyperarousal and did not significantly change sleep spindle properties in individuals with chronic insomnia disorder.39 Additionally, daridorexant 50 mg significantly reduced cumulative wake time versus placebo along the entire night, after both 1 and 3 months of treatment.40 In a post hoc analysis investigating the impact of daridorexant on wake bouts, wake bouts were defined as either short (≤6 minutes) or long (>6 minutes). When mean cumulative time spent in long or short wake bouts across the 8-hour PSG night were determined, it was shown that the increase in sTST observed with daridorexant versus placebo in Study 301 was underpinned by a reduction in time spent in long wake bouts.40 This decrease from baseline in time spent in long wake bouts was associated with an improvement in IDSIQ sleepiness domain scores.40
Participants who completed the 12-week Phase III studies were invited to enrol in a 40-week double-blind extension study.37 Participants originally randomised to daridorexant (10 mg, 25 mg, or 50 mg) remained on their respective treatments, while participants originally randomised to placebo were re-randomised to daridorexant 25 mg or placebo. In participants who originally entered Study 301, daridorexant 50 mg demonstrated persistent efficacy up to 1 year of treatment (exploratory analyses), as measured by mean change from baseline in sTST and improved daytime functioning (measured by mean change from baseline in IDSIQ total score).37
Safety analyses over the extension study showed a safety and tolerability profile similar to the initial Phase III studies.37 The overall incidence of treatment-emergent adverse events (TEAE) was similar across groups (35–40% for all daridorexant groups and placebo groups in the extension study). The incidence of serious TEAEs was <5.5% in all groups, and TEAEs leading to treatment discontinuation were generally similar between daridorexant groups (1.4–6.6%) versus placebo (4.7%), respectively.37 The most commonly reported TEAEs that started or worsened during the double-blind period with daridorexant 50 mg versus placebo were nasopharyngitis (8.0% versus 4.7%), accidental overdose (2.9% versus 0%), and somnolence (2.9% versus 0%), respectively.37 There was no evidence of excess next-morning sleepiness or rebound insomnia in participants who received daridorexant.37
Dauvilliers summarised that, during the clinical trial programme, daridorexant 50 mg was well tolerated, with no signs of dependency, improved sleep throughout the night, and improved daytime functioning with up to 1 year of treatment.36,37
Real-World Experience with Daridorexant Treatment in Chronic Insomnia Disorder
The European Insomnia Guidelines 2023 state that, while “the introduction of DORAs has probably been the most significant recent development in the pharmacological treatment of insomnia […] data remain to be validated through practical experience in everyday practice.”24 Liguori presented outcomes from four real-world studies with daridorexant that included patient populations with comorbid conditions or individuals who were already receiving alternative pharmacotherapy for insomnia and were switched to daridorexant.
Daridorexant Impact on Sleep and Quality of Life in Real-World Practice
In Germany, analysis of the Mainz Sleep Registry showed statistically significant improved sleep parameters (WASO, LPS, sTST, and TST) and HRQoL (measured using the European Quality of Life Visual Analogue Scale and European Quality 5 domain index) in individuals with insomnia disorder (n=32) treated with daridorexant 50 mg for up to 6 months.41,42
Daridorexant Treatment in Comorbid Insomnia and Sleep Apnoea
A study in the USA investigated daridorexant treatment in a cohort that included individuals with comorbid insomnia and sleep apnoea (COMISA).43 Liguori explained that COMISA is a highly prevalent and debilitating condition that is particularly challenging to diagnose and treat: 39–58% of individuals with obstructive sleep apnoea (OSA) experience insomnia symptoms, and 29–67% of individuals with insomnia meet diagnostic criteria for OSA.15
- Compared with those with insomnia or OSA alone, individuals with COMISA experience greater impairments in daytime functioning and HRQoL, and increased depressive and anxiety symptoms.15
- In this relatively small real-world study, treatment with daridorexant 25 mg or daridorexant 50 mg for ≥30 days reduced Insomnia Severity Index (ISI) for individuals with COMISA (n=15; change from baseline -6.9; p<0.01) and without COMISA (n=61; change from baseline -7.3; p<0.0001).43
- This study also reported results for individuals who switched to daridorexant from a prior insomnia pharmacotherapy (n=63/80, 78.8%). In this analysis, mean ISI score improved in the group of individuals who switched from any pharmacotherapy to daridorexant (n=63; 7.0±0.54 standard error of the mean [SEM]); p<0.0001) and in individuals who switched to daridorexant from zolpidem or eszopiclone (n=23; 7.6±0.86 [SEM], p<0.0001).43
Daridorexant Treatment in Individuals with Comorbid Insomnia and Psychiatric Disorders
A study from Pisa, Italy, reported outcomes with daridorexant 50 mg treatment for 3 months in a population that included individuals with comorbid unipolar/bipolar depression, anxiety disorders, or sedative hypnotic use or misuse (n=66).44 In this observational, uncontrolled study, insomnia, mood and anxiety symptoms were improved at Month 3 compared with baseline. Regression analysis showed that improvements in insomnia symptoms were associated with improvements in symptoms of comorbid conditions.44
A study from Rome, Italy, reported results from a population that included individuals with chronic insomnia disorder and comorbid depression, anxiety, or epilepsy (n=69).45 In this study, improvement in Clinical Global Impression (CGI-I) and Patient Global Impression (PGI-I) after 30 days of daridorexant 50 mg used as first drug chosen, add-on treatment, or switch from another drug was 58.0% for both outcomes. There was no difference between CGI-I and PGI-I according to the number of most common comorbidities, sex, number of previous medications, prior insomnia medication use, or insomnia duration.45 Of note, this study used a cross-tapering programme in patients under sedative hypnotics, in which these individuals reduced their sedative hypnotic dose by 25% each week over the course of 4 weeks whilst also receiving the full daridorexant 50 mg dose.45
On a related point, in a panel discussion following the presentations, García-Borreguero emphasised that, when switching a patient from benzodiazepine treatment to daridorexant, it is very important to slowly reduce the dose of benzodiazepine as the daridorexant treatment is started. This is because benzodiazepines have a strong rebound effect, meaning that insomnia symptoms may transiently worsen beyond baseline levels once benzodiazepine treatment is withdrawn. The tapering of the benzodiazepine dose should be done as slowly as possible; this may take weeks or even months.
Liguori summarised that real-world experience with daridorexant was associated with significant improvements in sleep quality in people with chronic insomnia disorder, including those with and without neurologic and psychiatric comorbidities. Daridorexant was also associated with improvements in sleep quality whether used as a first-ever treatment, treatment switch, or add-on therapy.42-45







