Meeting Summary
The annual Clinical Trials in Alzheimer’s Disease (CTAD) Congress brings together global experts to discuss developments in Alzheimer’s disease (AD) clinical research and showcase researchers’ work on AD trials. This article describes a recently initiated Phase II trial of a novel anti-tau monoclonal antibody, BMS-986446, the design for which was presented at the 17th CTAD Congress in Madrid, Spain, on 31st October 2024. BMS-986446 targets the microtubule binding region of the tau molecule to inhibit interneural transmission and prevent pathogenic spread of tau aggregates. The TargetTau-1 study (NCT06268886) will assess the efficacy of BMS-986446 by evaluating clinical, cognitive and functional decline, and tau deposition, and investigate the safety and tolerability profile of BMS-986446 in approximately 475 patients with early AD.
Introduction
A key pathological feature in Alzheimer’s disease (AD) pathology is neurofibrillary tangles, formed through aggregation of the microtubule-associated protein tau. Normal tau has a role in stabilising axonal microtubules; however, in pathological conditions, misfolding and hyperphosphorylation of tau leads to aggregation.1 Soluble tau aggregates can be transported from neuron to neuron, propagating tau pathology in different brain regions.1 Tau has long been considered a rational target for the development of treatments for AD. However, clinical trials of numerous drugs designed to interfere with tau pathology, including several monoclonal antibodies (mAbs) targeting the N-terminus of the tau protein, have been unsuccessful.2 Research efforts have moved towards targeting the microtubule binding region (MTBR), located in the mid-region of the protein, which is implicated in tau pathology: in vitro studies show that MTBR-containing tau species are a primary component of tau tangles, and that MTBR fibrils are readily taken up by neurons,3-5 while MTBR-tau levels in the cerebrospinal fluid (CSF) of patients with AD correlate with markers of AD clinical progression.6
BMS-986446 (formerly PRX005) is a first-in-class, humanised IgG1 kappa mAb that binds to the R1, R1, and R3 domains within the tau MTBR.7 Its proposed mechanism of action involves inhibition of interneural transmission to prevent the spread of tau pathology through neuronal circuits. In preclinical studies, BMS-986446 inhibited neuronal uptake of aggregated tau, reduced tau-induced neurotoxicity and promoted uptake of aggregated tau by phagocytic cells in vitro, and reduced the development of tau pathology in vivo.7 BMS-986446 also reduced the development of tau pathology in vivo and improved tau-related behavioural deficits in a murine model.7 A Phase I first-in-human study (NCT06084598)8 indicated that BMS-986446 was safe and well tolerated in healthy adults.9 Pharmacokinetic and pharmacodynamic analyses showed that BMS-986446 demonstrated dose-proportional exposure and achieved CSF concentrations sufficient for pharmacologic targeting.9
These studies support the development of BMS-986446 as a potential treatment for AD. This article describes a Phase II study designed to evaluate the efficacy, safety, and tolerability of BMS-986446 in patients with early AD.
Phase II Trial Design
TargetTau-1 (NCT06268886)10 is a global, randomised, double-blind, placebo-controlled, Phase II trial to evaluate the efficacy, safety, and tolerability of BMS-986446 in adults aged 50–80 years with early AD.10,11 The primary objective of the study is to evaluate clinical decline in patients treated with BMS-986446 compared with placebo. Clinical decline will be evaluated based on mean change from baseline in Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) score at 76 weeks (primary endpoint). Secondary objectives of the study include assessing the effects of BMS-986446 on brain tau deposition, measured using PET; determining whether treatment with BMS-986446 delays cognitive and functional decline, assessed using a selection of rating scales (Figure 1); and evaluating safety. Exploratory endpoints include measures of BMS-986446 pharmacokinetics and immunogenicity, plasma biomarkers of AD, brain MRI changes, and quality of life assessments.11
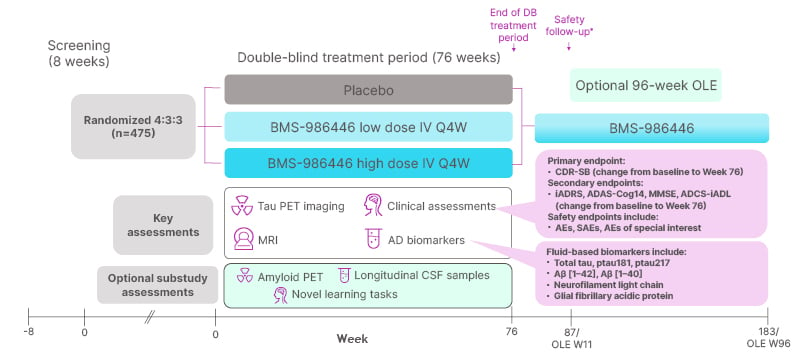
Figure 1: TargetTau-1 study design.11
*A safety follow-up visit is required 11 weeks after the last dose of blinded study drug for all participants who do not enter the OLE.
Aβ: amyloid-β; AD: Alzheimer’s disease; ADCS-iADL: Alzheimer’s Disease Cooperative Study-Instrumental Activities of Daily Living Scale; AE: adverse event; CDR-SB: Clinical Dementia Rating Scale Sum of Boxes; CSF: cerebrospinal fluid; DB: double-blind; iADRS: Integrated Alzheimer’s Disease Rating Scale; IV: intravenous; MMSE: Mini-Mental State Examination; OLE: open-label extension; Q4W: every 4 weeks; SAE: serious adverse event; W: week.
Study sites in 10 countries in the USA, Europe, and the Asia–Pacific region will enrol an estimated 475 patients aged 50–80 years, with a diagnosis of mild cognitive impairment due to AD or mild AD dementia consistent with the National Institute on Aging and Alzheimer’s Association (NIA-AA) core clinical criteria.10,12 Patients must also have AD pathology confirmed by plasma biomarkers and tau PET imaging, mini-mental state exam (MMSE) score of 22–30, and objective episodic memory impairment. Patients will not be permitted to enter the study if they have a diagnosis of another neurologic condition that could contribute to cognitive impairment, a psychiatric diagnosis or symptoms, or pathologic findings on brain MRI that could interfere with the study. Participants enrolled in the study will be randomised 4:3:3 to placebo, low-dose BMS-986446, or high-dose BMS-986446 (Figure 1). BMS-986446 (or matched placebo) will be administered by intravenous infusion every 4 weeks for 76 weeks. At the end of the double-blind treatment period, patients will have the option to enter a 96-week open-label extension.11
Patients who consent to lumbar puncture for CSF sampling are eligible to participate in a biomarker substudy, which aims to generate a multi-modal dataset tying biological changes with clinical benefit.11 Alongside longitudinal measurement of CSF biomarkers and BMS-986446 levels, these patients will undertake learning tasks (Figure 1) designed to provide an objective measure of cognitive function with discriminatory power to detect changes in brain function in early AD. Patients in the substudy will also undergo additional PET scans to measure amyloid deposition (Figure 1), to determine whether brain tau and amyloid-β change in tandem in response to an anti-tau mAb. This will inform future investigation of combination versus monotherapy strategies for optimal clearance of amyloid plaques and neurofibrillary tangles.
Trial Progress and Outlook
Enrolment to the TargetTau-1 trial began in March 2024. The estimated study completion date is November 2027.10 Results of this study will determine further clinical development of BMS-986446 as a potential treatment for AD.







