Meeting Summary
Nipocalimab, a high-affinity, fully human, aglycosylated, effectorless, monoclonal antibody, has been developed as an add-on treatment for myasthenia gravis (MG). The agent, administered every 2 weeks, aims to selectively block neonatal Fc receptor (FcRn), in order to reduce levels of circulating immunoglobulin G (IgG) including autoantibodies and ameliorate disease manifestations, while preserving immune function.
In this poster presentation, Carlo Antozzi outlined top-line results from the 24-week, double-blind, placebo-controlled, randomised Vivacity-MG3 Phase III study (NCT04951622).1 Nipocalimab, he explained, demonstrated efficacy and safety in a broad antibody-positive population, suggesting it could provide the first FcRn treatment option with a predictable dosing schedule in generalised MG (gMG).
Condition of Unmet Need
MG is a rare IgG autoantibody-mediated disease, characterised by fluctuating weakness of the voluntary muscles.2,3 The weakness, which tends to worsen with activity,3 is caused by the pathogenic IgG-mediated disruption of cholinergic transmission at the neuromuscular junction.2 In ocular MG, the weakness only affects the extraocular muscles, whereas gMG affects the bulbar muscle, extremities, or axial muscles.3
gMG is an area of unmet medical need. Although therapies, including cholinesterase inhibitors and immunosuppressants, are available, many people still experience symptoms, exacerbations, and reduced quality of life.2 In addition, such agents may be associated with side effects that can limit tolerability.2
Blocking FcRn: A New Approach
Nipocalimab is an investigational fully human monoclonal antibody designed to bind with high affinity and selectively block FcRn, reducing levels of circulating IgG antibodies, including autoantibodies, while also preserving immune function.4 As such, it is a targeted approach not resulting in broad immunosuppression.4 Developed as an add-on treatment for MG to be administered every 2 weeks, nipocalimab could ameliorate disease manifestations, said Antozzi.
In a Phase II study, intravenous (IV) nipocalimab was shown to be generally well tolerated, and there was evidence of a dose-dependent reduction in MG Activities of Daily Living (MG-ADL) total score after 57 days.2 Vivacity-MG3, a Phase III, multicentre, randomised, double-blind, placebo-controlled study (NCT04951622),1 aimed to build on these findings. Top-line results from the 24-week double-blind phase were presented at the 10th Congress of the European Association of Neurology (EAN).5 In this study, nipocalimab demonstrated efficacy and safety in a broad antibody-positive population, including acetylcholine receptor (AChR+), muscle-specific tyrosine kinase (MuSK+), and low-density lipoprotein receptor 4 (LRP4+) positive patients.5
Vivacity-MG3 Phase III Study5
The study enrolled 153 adults with seropositive Myasthenia Gravis Foundation of America (MGFA) Class IIa–IVb gMG who had experienced an inadequate response (MG-ADL ≥6) to stable standard of care (SOC) treatment (see Table 1 for baseline characteristics). Of these, 77 patients (63 AChR+; 12 MuSK+; 2 LRP4+) were randomised to IV nipocalimab plus SOC, and 76 patients (71 AChR+; 4 MuSK+; 1 LRP4+) to IV placebo plus SOC, for 24 weeks in the double-blind phase. A loading dose was administered at Week 0, after which patients were treated every 2 weeks. The primary endpoint was improvement in MG-ADL total score to Week 24, with improvement of Quantitative Myasthenia Gravis (QMG) total score and MG-ADL responder rates being key secondary endpoints.
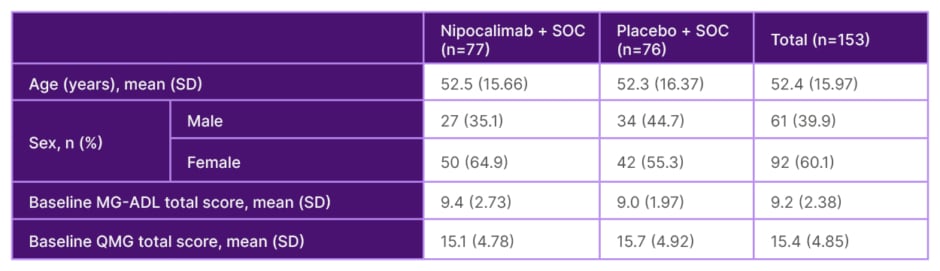
Table 1: Phase III Vivacity-MG3 study baseline characteristics.5
MG-ADL: Myasthenia Gravis Activities of Daily Living; QMG: Quantitative Myasthenia Gravis; SD: standard deviation; SOC: standard-of-care.
The double-blind phase was followed by an open-label phase of variable duration (ongoing), during which all participants will receive SOC plus nipocalimab every 2 weeks, and a safety follow-up of a further 2 weeks.
Results
The study recorded a statistically significant improvement from baseline in MG-ADL (Figure 1A, primary endpoint) and QMG (Figure 1B, first key secondary endpoint) in the nipocalimab arm, compared to the placebo arm.5 In the nipocalimab group, the mean (standard error [SE]) change in MG-ADL from baseline to the averaged total score across Weeks 22, 23, and 24 was -4.7 (0.33), compared to -3.25 (0.34) in the placebo group. The difference in least squares (LS) means (SE) was -1.45 (0.47), p=0.002. The mean (SE) QMG change from baseline over Weeks 22 and 24 was -4.86 (0.50), compared to -2.05 (0.50) in the placebo group. The difference in LS means (SE) was -2.81 (0.71), p<0.001. MG-ADL responder rate (percentage of patients with ≥2-point improvement on average change of MG-ADL, second key secondary endpoint) at Weeks 22–24 was 68.8% in the nipocalimab group, and 52.6% in the placebo group (p=0.021). In addition, more patients achieved sustained response from Weeks 4–24 with nipocalimab compared to placebo. At Weeks 22–24, 46.8% of those in the nipocalimab group saw a ≥50% improvement in MG-ADL, compared to 25% in the placebo group (Figure 2).
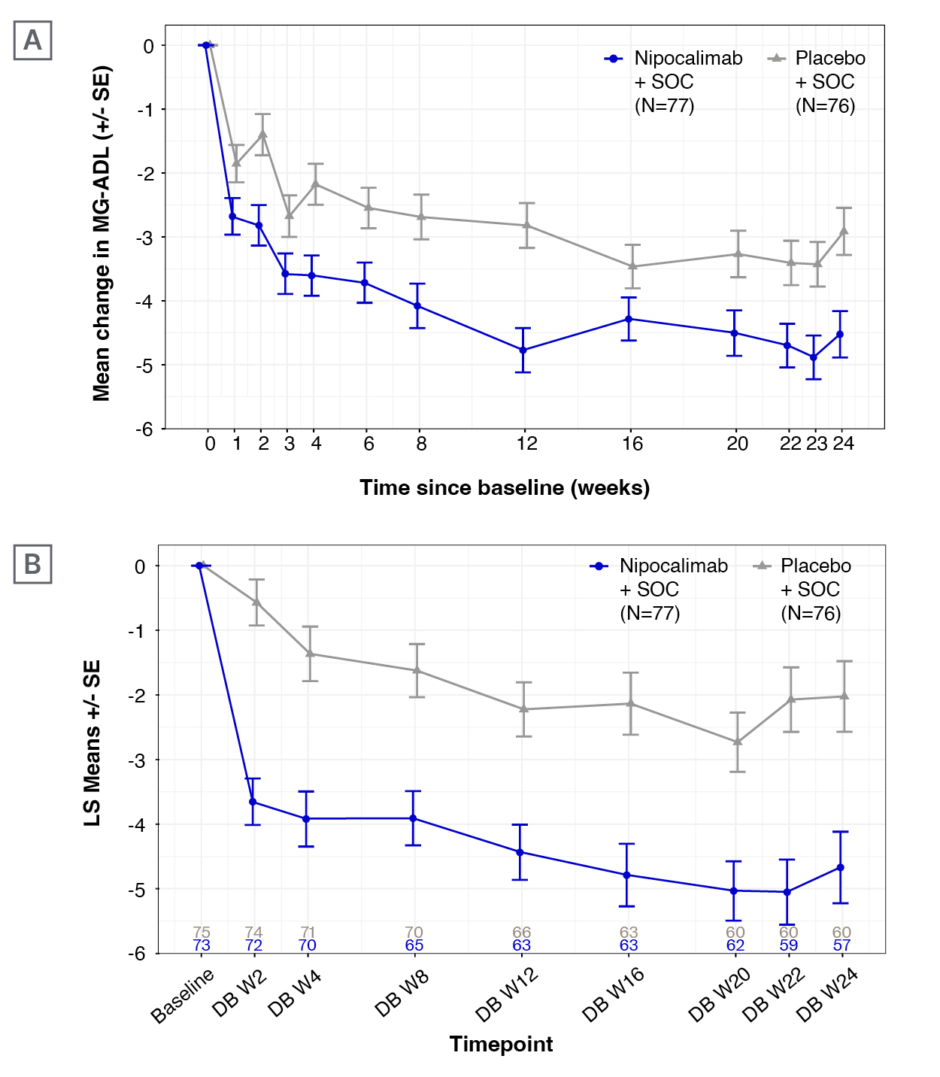
Figure 1: A) Mean (±standard error) change in Myasthenia Gravis Activities of Daily Living (MG-ADL) and B) Quantitative Myasthenia Gravis (QMG) over 24 weeks of treatment in the Vivacity-MG3 study.5
LS: least squares; MG-ADL: Myasthenia Gravis Activities of Daily Living; QMG: Quantitative Myasthenia Gravis; SE: standard error; SOC: standard-of-care; W: Week.
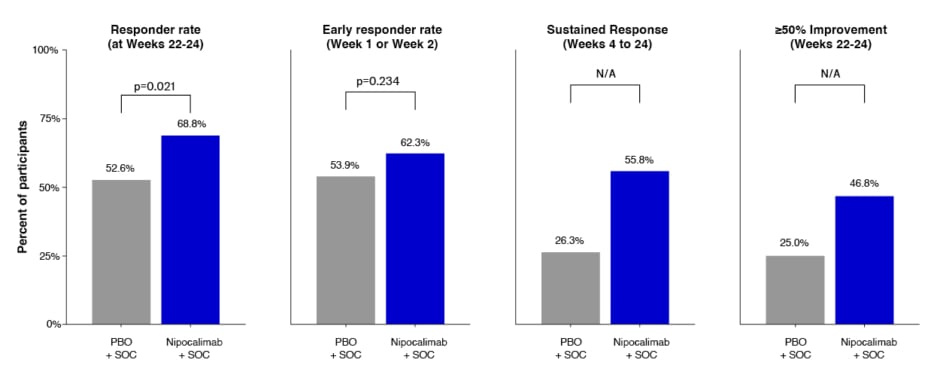
Figure 2: Key secondary MG-ADL responder endpoints.5
*Responder rate: percentage of patients with ≥2-pt improvement on average change of MG-ADL over Weeks 22, 23, and 24. Early responder rate: percetnage of patients with ≥2-pt improvement on MG-ADL at Week 1 or Week 2. Sustained response: at least 2-pt improvement from Weeks 4–24 with no more than two non-consecutive excursions from Weeks 6–23. 50% symptom improvement: percetnage of patients with ≥50% improvement on average change of MG-ADL over Weeks 22, 23, and 24. P-values from CMH test controlling for baseline MG-ADL total score (<9, ≥9), autoantibody status, and region. N/A: Test not performed because a preceding comparison was not statistically significant at 2-sided alpha=0.05.
CMH: Cochran-Mantel-Haenszel; MG-ADL: MG Activities of Daily Living; N/A: not applicable; PBO: placebo; pt: point; SOC: standard-of-care.
During the double-blind phase, around one-third (31.2%; 24/77) of patients receiving nipocalimab achieved the pre-specified endpoint of minimal symptom expression, defined as an MG-ADL total score of 0 or 1, at any point. Overall, 13.2% (10/76) of those in the placebo arm achieved minimal symptom expression.
Nipocalimab was generally well tolerated in this 6-month study. In the treatment group, 81.6% (80/98) of patients experienced an adverse event (AE) and 9.2% (9/98) experienced a serious AE, compared to 82.7% (81/98) and 14.3% (14/98) in the placebo group, respectively. The most common AEs were headache (14/98 [14.3%] in the treatment group; 17/98 [17%] in the placebo group), muscle spasms (12/98 [12.2%] in the treatment group; 3/98 [3.1%] in the placebo group), and COVID-19 (15/98 [15.3%] in the treatment group; 12/98 [12.2%] in the placebo group). Infusion reactions were also common, affecting 10 patients (10.2%) in the treatment group, and 11 (11.2%) in the placebo group.
Key Learnings
Summing up, Antozzi said Vivacity-MG3 was the first registrational study of an FcRn blocker to show sustained efficacy and a tolerable safety profile through 6 months of dosing.







