Abstract
Background: Recognising acute stroke symptoms is crucial in providing timely treatment. However, evidence suggests that females often experience unique symptoms compared with males, resulting in delays to seeking medical attention and treatment. This systematic review and meta-analysis evaluated whether sex is associated with differences in acute stroke symptoms.
Methods: Searches from 1946 to 7th September 2021 were carried out using MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane Library. Studies reporting acute stroke symptoms in adult females and males were eligible for inclusion. Eleven observational studies met the inclusion criteria. Methodological quality was assessed using the Newcastle–Ottawa scale (NOS). Data were meta-analysed using a random-effects model.
Results: Compared with males, females had higher odds of experiencing headache (odds ratio [OR]: 1.27; 95% confidence interval [CI]: 1.01–1.59); change in level of consciousness (OR: 1.36; 95% CI: 1.13–1.63); fatigue (OR: 1.53; 95% CI: 1.04–2.25); and incontinence (OR: 1.44; 95% CI: 1.29–1.60). In contrast, females were at lower odds of experiencing trouble speaking (OR: 0.79; 95% CI: 0.64–0.96); trouble walking, loss of balance, or co-ordination (OR: 0.55; 95% CI: 0.39–0.76); and dizziness (OR: 0.77; 95% CI: 0.64–0.94) compared with males. No difference was found in confusion, difficulty understanding speech, trouble seeing in one or both eyes, mental status change, and nausea or vomiting.
Discussion: Sex differences do exist in some acute stroke symptoms. At the same time, the overlap in symptoms between sexes was substantial. Healthcare professionals and public health campaigns should continue to promote classic symptoms of acute stroke, whilst taking into account the less common symptoms and the potential differences in symptoms experienced by females and males.
Key Points
1. As recently as 2020, the World Health Organisation (WHO) published data showing that acute strokes were the second leading cause of death worldwide.2. This systematic review and meta-analysis of 11 studies of 15,465 patients with acute stroke revealed considerable overlap in symptomology between male and female patients for both ‘traditional’ and ‘non-traditional’ symptoms, suggesting these terms are outdated.
3. While significant sex differences in stroke presentations weren’t found by this analysis, better community knowledge of atypical symptoms of acute stroke could improve earlier hospital presentation for affected patients, regardless of sex.
INTRODUCTION
In recent years, acute stroke mortality has decreased due to advancements in treatment, lifestyle changes, and an increased focus on primary prevention; however, rates are still considerably high globally.1 In 2020, the World Health Organization (WHO) published data that showed acute stroke to be the second leading cause of deaths at a global level in 2019, instigating approximately 11% of the total deaths.2 This number is set to rise exponentially due to ageing and growth of the population, as well as improvements in treatment leading to better survival rates.3,4
Recognising acute stroke symptoms is crucial in providing timely treatment. Patients who receive rapid treatment are more likely to have better outcomes and recovery, even if the initial stroke is relatively severe.5 As a result, establishing the time of onset of symptoms is imperative.
Previous studies have reported that acute stroke symptomology can differ between females and males, leading to differences in acute stroke outcomes.6 In particular, females have been documented to present with different, unique symptoms of acute stroke rather than those considered ‘traditional’ or ‘typical’ (e.g., numbness and weakness).7,8 These unique symptoms are often labelled as ‘non-traditional’ or ‘atypical’, and cover a broad spectrum.8 To date, there is no consensus as to which symptoms can specifically be classified as ‘non-traditional’. This lack of consensus means that these unique symptoms are often not recognised as acute stroke symptoms, and their manifestation can result in delays to medical attention, under-diagnosis, and under-treatment, which in turn can exacerbate adverse outcomes of acute stroke.9
Potential differences in acute stroke symptoms can contribute towards the delay in seeking medical assistance in females, making diagnosis more difficult and delaying effective treatment or therapy.10 Current campaigns to increase awareness are based on typical or traditional symptoms of acute stroke.10-12 In order to minimise the time between symptom onset and seeking medical attention, a more sustainable public health campaign needs to be established and addressed through theory-based interventions targeting the entire range of acute stroke symptoms, as opposed to the most common.13
A systematic review was conducted to understand better if and how acute stroke symptoms differ between sexes. Such information is required for the development of effective health campaigns to educate both the public and healthcare professionals on recognising alternative symptoms of acute stroke and prompt further research.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in all aspects of this systematic review.14 This study was submitted for registration on the International Prospective Register of Systematic Reviews (PROSPERO), under registration number CRD42020220866.
Search Strategy
Keywords and medical subject headings related to acute stroke, ischaemic stroke, haemorrhagic stroke, cerebrovascular accident, sex, and symptoms were identified.
Searches were carried out on MEDLINE, Embase, CINAHL, and the Cochrane Library. The following search strategy was employed on the OVID platforms and modified for other databases:
(((sex OR gender) adj2 (distribution OR different* OR dispuarit* OR factor* OR inequality* OR specific))) OR (((male* or men) and (female* or women)).ti.) AND (((stroke) adj2 (ischaemic OR intracerebral OR intraparenchymal OR subarachnoid OR haemorrhage*)) OR (cerebrovascular accident OR CVA OR brain attack)) AND (symptom* OR presenting OR presentation OR sign* OR character* OR manifestation)
Grey literature searches were conducted using OpenGrey and ProQuest-Digital Dissertations and Theses.
Eligibility Criteria
Original, quantitative studies (randomised or quasi-randomised controlled trials), interventional studies, and observational studies (cohort, cross-sectional, and case-control studies) that reported on acute symptom presentation in females and males aged ≥18 years presenting to hospital with acute stroke, as confirmed by a physician or neurologic examination and/or radiological scan, were included. Acute stroke was defined as a clinical syndrome, consisting of rapidly developing clinical signs of focal (or at times global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin, including either ischaemic or haemorrhagic stroke. Outcomes of interest were traditional and non-traditional symptoms of acute stroke.
Traditional acute stroke symptoms were identified using the five main categories, which are classified by the Centers for Disease Control and Prevention (CDC) as sudden numbness or weakness in the face, arm, or legs, especially on one side of the body; sudden confusion, trouble speaking, or difficulty understanding speech; sudden trouble seeing in one or both eyes, sudden trouble walking, dizziness, loss of balance, or lack of co-ordination; and sudden, severe headache.7
Further to this, the following symptoms commonly classified as non-traditional were identified from existing literature: general weakness; pain, including facial or hemi-body pain; change in level of consciousness; mental status change; fatigue; nausea or vomiting; and incontinence.8,15-17
Studies were not limited by date of publication. Diagnostic studies, qualitative studies, editorials, commentaries, review articles, case reports, and letters without quantitative data were not included. Only studies in English were included due to resource limits. As transient ischaemic attacks do not typically cause lasting damage, they are often seen as a precursor to acute stroke rather than an acute stroke subtype.18 Therefore, studies that reported symptoms of transient ischaemic attacks were excluded. Conference abstracts and reviews were also excluded.
Risk of Bias
Due to the observational methodology of the included papers, the quality assessment was based on the Newcastle–Ottawa scale (NOS).19
Data Extraction
The following data were extracted from all eligible studies: study participants’ demographic information; study characteristics, including design, methodology, and geographical location; sample size; eligibility criteria; diagnostic criteria; the proportion of females and males with acute stroke symptoms; and the proportion of females and males with each symptom, both traditional and non-traditional.
Terminologies or symptoms that were considered synonymous with the symptoms identified in the outcomes were combined. Where studies grouped symptoms using an umbrella term, such as ‘speech symptoms’ or ‘visual deficits’ without further description or definition, this information was not extracted as it was not possible to disaggregate which symptoms were included. Data were combined if one symptom or another was reported such as ‘nausea or vomiting’. Where studies reported one symptom and another, such as nausea and vomiting, the prevalence of both were extracted where possible.
Statistical Analysis
Effect measures were expressed as odds ratios (OR) with 95% confidence intervals (CI) to formally test the female–male differences in the prevalence of acute stroke symptoms. The OR was the ratio of the odds of an event, such as an acute stroke symptom, occurring in males to the odds of the event occurring in females. An OR of 1 implied that a symptom was experienced equally in both females and males; an OR of >1 implied that the event was more likely in females; and a ratio of <1 implied that the event was less likely in females.20 As a certain degree of heterogeneity was expected in the observational studies included in this review in terms of study designs, patient populations, data analysis methods, and outcomes, a Mantel–Haenszel model for random effects was used.21,22 A p value of <0.05 was considered statistically significant.21
Statistical heterogeneity was assessed using the Chi-squared test (significance level of 0.1) and I2 statistic. I2 values of <40% were considered to represent low heterogeneity, 40–60% moderate heterogeneity, 60–90% substantial heterogeneity, and >90% as considerable heterogeneity.22
Sub-group analyses were not performed as none of the meta-analyses included at least 10 studies for each characteristic modelled and, therefore, were unlikely to produce useful findings.22 Similarly, it was established a priori that funnel plots would be used to assess for publication bias when 10 or more studies were combined. None of the analyses combined a sufficient number of studies to produce such funnel plots; therefore, the effects of publication bias could not be determined.
All statistical analyses were performed using Review Manager (Cochrane, London, UK), version 5.4.1 for Mac.23
RESULTS
Study Selection
The literature search returned 2,877 results in total. Search results from each database were deduplicated. Two investigators independently screened the titles and abstracts of the studies. Any discrepancies in study assignments were solved through discussion and consensus between the two investigators. If no consensus could be reached, a third-party reviewer was available for consultation. In total, 11 studies fulfilled the inclusion criteria and were included in the meta-analysis. Figure 1 summarises the study selection process.
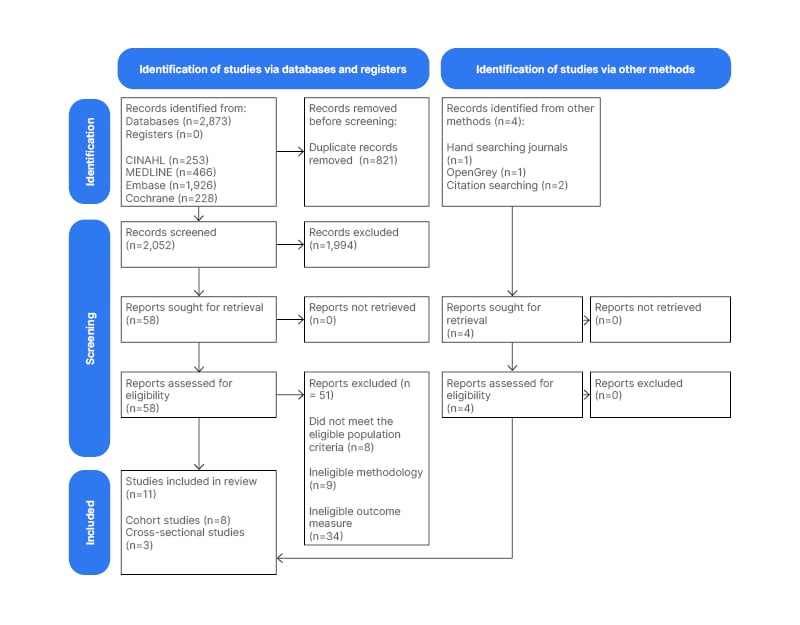
Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of selection of studies for inclusion in the review.14
Characteristics of Included Studies
Eight cohort studies and three cross-sectional studies involving 15,465 individuals in total were included, 7,763 (50.2%) of whom were female and 7,702 (49.8%) were male. Study participants were all recruited from hospitals from 13 countries, predominantly from the USA (n=4). The years during which data were collected ranged between 1985–2010. The mean age of female participants was 71 years, while the mean age of male participants was 65. Three of the studies included patients presenting with ischaemic stroke only. One study investigated patients with haemorrhagic stroke only. The remaining seven studies included both ischaemic and haemorrhagic stroke patients. The full study characteristics are summarised in Table 1.
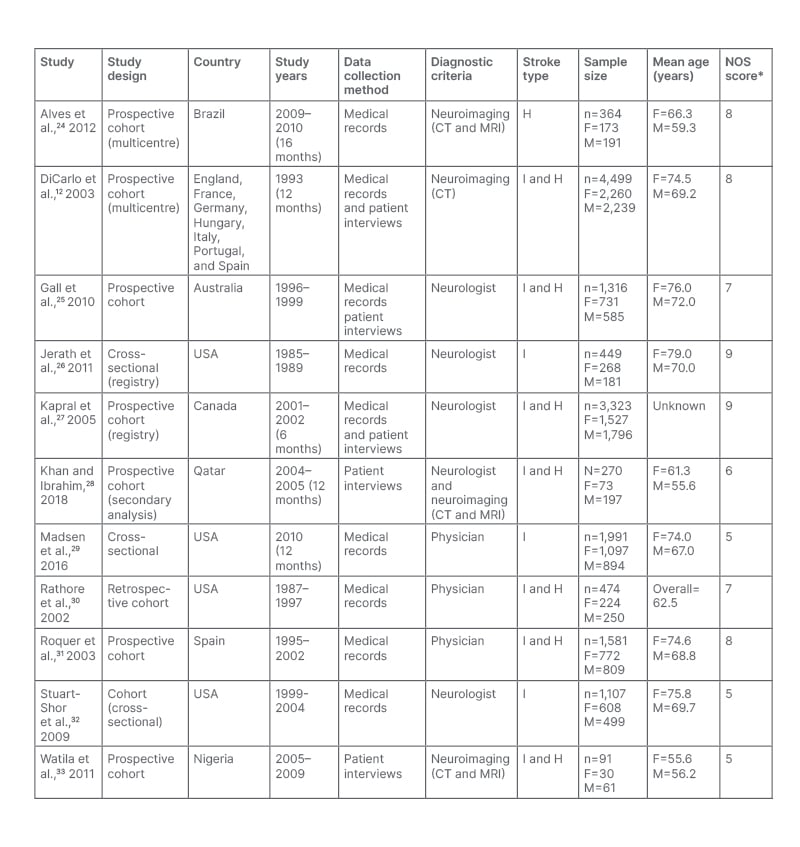
Table 1: Summary of characteristics of included studies.
The overall population age (years) where individual mean age for females and males is not stated.
*The NOS was out of a maximum of 9 stars. NOS 0–3: very high risk of bias (low quality); 4–6: high risk of bias; and 7-9: low risk of bias (high quality).
F: female H: haemorrhagic stroke; I: ischaemic stroke; M: male; NOS: Newcastle–Ottawa scale.
Risk of bias in studies
The quality of the eight included cohort studies was variable, whilst the quality of the three included cross-sectional studies was considered good.
Results of syntheses
Table 2 provides the pooled crude ORs for sex differences in each examined symptom of patients presenting with acute stroke.
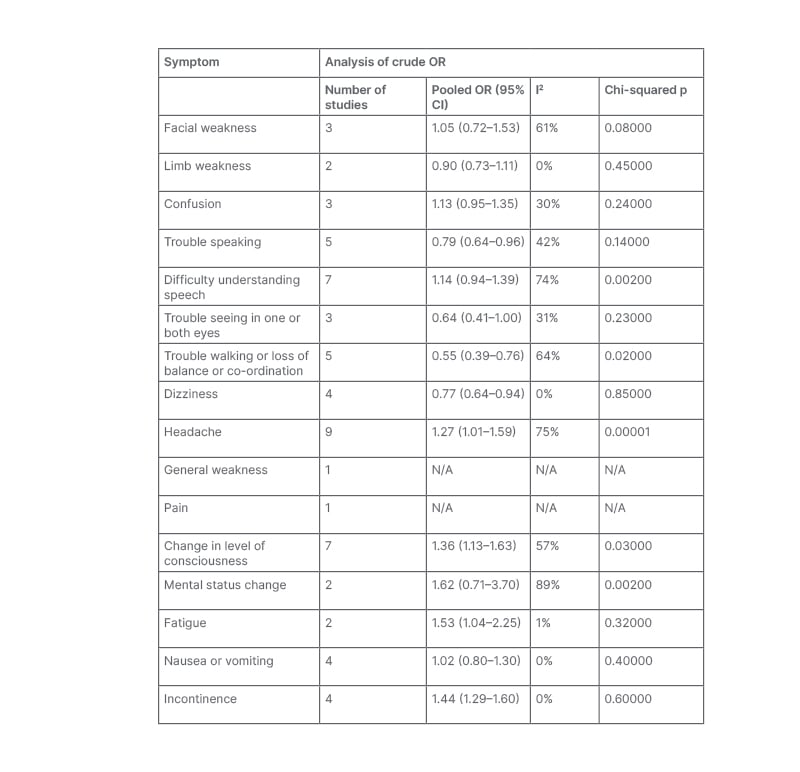
Table 2: Aggregated meta-analysis for all included symptoms of acute stroke.
CI: confidence interval; N/A: not applicable; OR: odds ratio.
Numbness or weakness in the face, arms, or legs, especially on one side of the body due to the variability in reported symptoms for this outcome, it was deemed inappropriate to pool data between all four studies that reported ‘weakness’ as a symptom.25,28,30,33 Instead, symptoms that more than one study reported data for were identified and analysed, including facial weakness and limb weakness.
No significant sex difference was noted in presentation with facial weakness (OR: 1.05; 95% CI: 0.72–1.53).25,30,33 There was a significant, moderate level of statistical heterogeneity between these studies (I2: 61%; Chi-squared: 5.07; p=0.08).
Similarly, no significant sex difference was found in presentation with limb weakness (OR: 0.90; 95% CI: 0.73–1.11).25,28 No heterogeneity was noted (I2: 0%; Chi-squared: 0.58; p=0.45). This may be due to the size of this meta-analysis; it is not always possible to estimate heterogeneity with much precision in small meta-analyses.34
Confusion
No significant sex difference was noted in the overall pooled OR for confusion (OR: 1.13; 95% CI: 0.95–1.35).12,25,26 A low but non-significant level of heterogeneity was noted between these studies (I2: 30%; Chi-squared: 2.85; p=0.24).
Trouble speaking
Females had a significantly lower odds of presenting with trouble speaking than males (OR: 0.79; 95% CI: 0.64–0.96).12,25,26,28,33 The I2 value of 42% suggested a moderate level of heterogeneity between these studies, but this was not statistically significant (Chi-squared: 6.90; p=0.14).
Difficulty understanding speech
Overall, there was no significant sex difference in presentation with symptoms that resulted in difficulty understanding speech (OR: 1.14; 95% CI: 0.94–1.39).12,25,26,28,31,33 Statistical heterogeneity was substantial, as evidenced by the I2 value of 74% and statistically significant Chi-squared test results (Chi-squared: 18.99; p=0.002).
Trouble seeing in one or both eyes
Overall, there was no significant sex difference in presentation with the symptom of trouble seeing in one or both eyes (OR: 0.64; 95% CI: 0.41–1.00).26,30,33 Heterogeneity was low and non-significant (I2: 31%; Chi-squared: 2.90; p=0.23).
Trouble walking or loss of balance or co-ordination
Overall, females had significantly lower odds of presenting with the symptom of trouble walking or loss of balance or co-ordination than males (OR: 0.55; 95% CI: 0.39–0.76).25,26,28,30,32 There was a significant, moderate level of statistical heterogeneity between these studies (I2: 64%; Chi-squared: 11.21; p<0.02).
Dizziness
Females had significantly lower odds of presenting with dizziness than males (OR: 0.77; 95% CI: 0.64–0.94).25,26,29,30 Low heterogeneity was noted; this was non-significant (I2: 0%; Chi-squared: 0.82; p=0.5).
Headache
Females with acute stroke had significantly lower odds than males of presenting with headache (OR: 1.27; 95% CI: 1.01–1.59).24-28,30,32,33 The I2 value of 75% and significant Chi-squared statistic of 31.54 (p=0.0001) suggested a substantial level of statistical heterogeneity between these studies.
General weakness
Only one study reported general weakness as a symptom of acute stroke in females and males; therefore, a pooled OR could not be calculated.26 The OR calculated from the prevalence of females and males who experienced general weakness on presentation with acute stroke in this study showed that females had significantly higher odds of presenting with general weakness than males (OR: 1.84; 95% CI: 1.19–2.85).
Pain
Only one study that reported pain as an acute stroke symptom;therefore, a pooled OR could not be calculated.26 The OR calculated from the descriptive statistics provided in the study showed no significant sex difference in presentation with pain in acute stroke (OR: 0.57; 95% CI: 0.27–1.20).
Change in level of consciousness
Overall, females had significantly higher odds of presenting with a change in level of consciousness than males (OR: 1.36; 95% CI: 1.13–1.63).12,24,25,27-29,33 Statistical heterogeneity was moderate as evidenced by the I2 value of 57% and statistically significant Chi-squaredtest results (Chi-squared: 13.83; p=0.03).
Mental status change
Overall, there was no significant sex difference in presentation with mental status change as an acute stroke symptom (OR: 1.62; 95% CI: 0.71–3.70).26,32 The I2 value of 89% and significant Chi-squared statistic of 9.17 (p=0.002) suggested a substantial level of statistical heterogeneity between these studies, most likely because of the small size of the meta-analysis.34
Fatigue
Overall, females had significantly higher odds than males of presenting with fatigue (OR: 1.53; 95% CI: 1.04–2.25).26,32 Low heterogeneity was noted; this was not significant (I2: 1%; Chi-squared: 1.01; p=0.32). As noted above, this may be due to the size of this meta-analysis.34
Nausea or vomiting
Overall, no significant sex difference in presentation with nausea or vomiting as an acute stroke symptom was found (OR: 1.02; 95% CI: 0.80–1.30).25,26,32,33 Low heterogeneity was noted, but this was not significant (I2: 0%; Chi-squared: 2.98; p=0.40).
Incontinence
Overall, females with acute stroke had higher odds of presenting with incontinence than males (OR: 1.44; 95% CI; 1.29–1.60).12,25,26,28 No significant heterogeneity was observed (I2: 0, Chi-squared: 1.86; p=0.60).
DISCUSSION
This systematic review and meta-analysis of 11 studies, including 15,465 patients, shows that sex differences exist in some acute stroke symptoms. At the same time, overlap in symptoms between females and males with acute stroke was substantial.
A causal relationship between sex and acute stroke symptoms cannot be inferred from these results, particularly as age, ethnicity, and stroke subtype may have had a confounding effect. Moreover, these results are not indicative of sex differences in traditional acute stroke symptoms commonly known by the public and healthcare professionals. However, these results do provide evidence to suggest that females are more likely to present with non-traditional symptoms than males, with considerable overlap of traditional symptoms between both sexes. This is important for the applicability of public awareness campaigns to increase recognition of acute stroke in the community, irrespective of sex. It is imperative to continue promoting the awareness of traditional or classic acute stroke symptoms, as they appear to present in females and males. However, both the public and healthcare professionals should also be aware of the possibility of less commonly known symptoms of acute stroke manifesting, such as headache, change in level of consciousness, fatigue, and incontinence, so as not to delay treatment.
Implications for Future Research and Clinical Practice
Based on these findings, researchers and healthcare professionals should use caution when labelling acute stroke symptoms as traditional or non-traditional or ‘female/male-specific’ and instead focus on the established differences and overlap in symptom presentation between females and males. Furthermore, these results demonstrate the potential to revise national and international public health campaigns to highlight less common acute stroke symptoms and the possible differences in acute stroke symptom presentation between women and men. Alongside this, healthcare professionals need to be educated to recognise and explain these sex differences in acute stroke symptoms to patients presenting with acute stroke and those identified as at risk of acute stroke. Given the importance of age and ethnicity as explanatory factors, it is recommended that any future studies adjust for age and ethnicity, testing for interactions and adjusting for risk factors in a standardised way to allow for comparisons between studies.
Strengths and Limitations
This is the first systematic review and meta-analysis to examine sex differences in acute stroke symptoms. Scrupulous searching meant that relevant studies were identified; hence, results and conclusions of this systematic review are likely to be based on a synthesis of the available evidence. In addition to this, a rigorous risk of bias assessment was performed to help establish transparency of evidence synthesis results and findings.
As well as strengths, there are also limitations to this systematic review and meta-analysis to take into consideration. First, a limited number of publications looked into sex differences in acute stroke symptoms, rather than just sex differences in acute stroke outcomes or severity, representing a limitation within the current body of evidence. Secondly, the investigators could only read English; therefore, only studies published in English were included, which may have excluded potentially relevant articles. Finally, comparability of individual studies was hampered by the use of different definitions and combining of stroke symptoms in individual studies.
CONCLUSIONS
This systematic review and meta-analysis show that whilst there may be sex-related differences in some acute stroke symptoms, there is also significant overlap between the sexes in others. Moreover, there is little evidence to indicate sex differences in traditional and non-traditional acute stroke symptoms, suggesting that these terms are outdated. Instead, the data from this systematic review and meta-analysis suggest that public health campaigns and professionals should continue to focus on the classic symptoms of stroke for both sexes whilst also reflecting a broader spectrum of possible symptoms. Potential differences in symptom presentation between females and males should be taken into account to aid early recognition and avoid delays in treatment. Future studies should aim to shed light on the relationship between age, sex, ethnicity, and acute stroke symptoms.