Abstract
Adult-onset idiopathic focal dystonia is the most common type of primary dystonia, and adult-onset idiopathic cervical dystonia (AOICD) is its most prevalent phenotype. AOICD is an autosomal-dominant disorder with markedly reduced penetrance; clinical expression is dependent on age, sex, and environmental exposure. Motor symptoms at presentation are poorly recognised by non-specialists, leading to long delays in diagnosis. Certain features of history and examination can help diagnose cervical dystonia. There is a relatively high prevalence of anxiety and/or depression, which adversely affects health-related quality of life. Recent studies indicate that patients with AOICD also have disordered social cognition, particularly affecting emotional sensory processing. AOICD can be treated reasonably effectively with botulinum toxin injections, given at 3-month intervals. Oral antidystonic medications are often trialled initially, but are largely ineffective. Comprehensive modern management of patients with AOICD requires recognition of presence of mood disorders, and actively treating the endogenous mood disorder with antidepressant therapy. Botulinum toxin injections alone, no matter how expertly given, will not provide optimal therapy and improved health-related quality of life without an holistic approach to patient management. Increasing evidence indicates that AOICD is a neurophysiological network disorder of GABAergic inhibition, causing a syndrome of dystonia, mood disturbance, and social cognitive dysfunction, with the superior colliculus playing a central role.
Key Points
1. Cervical dystonia typically presents to neurologists as a motor disorder but has a spectrum of non-motor symptoms, including psychiatric, sensory, and cognitive function. The non-motor symptoms can have a significant impact on quality of life.2. Botulinum toxin is an effective treatment but a good knowledge of anatomy and careful muscle selection is needed for effective outcomes.
3. Although classically considered a basal ganglia disorder, it is likely that superior colliculus dysfunction underlies the motor and non-motor symptoms.
INTRODUCTION
Dystonias are a group of hyperkinetic movement disorders characterised by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous.1 They can be classified into primary (idiopathic or genetic) or secondary. Anatomical distribution of the dystonia can be focal, multifocal, segmental, or generalised.2
Adult-onset idiopathic focal dystonia (AOIFD) is the most frequent presentation of dystonia with a number of phenotypes; their typical manifestations are shown in Table 1. Sex is probably the single most important biological variable in relation to disease penetrance and expression. In AOIFD, the female to male sex ratio increases with increasing age of onset, which accounts for 60% of the variation in sex ratios.3,4
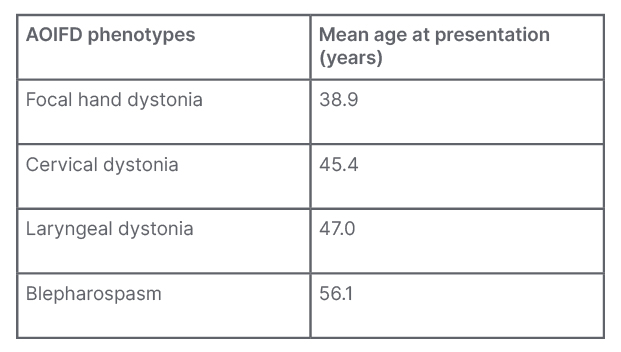
Table 1: The spectrum of presentations with adult-onset idiopathic focal dystonia.
The only phenotype with a male predominance is focal hand dystonia.
AOIFD: adult-onset idiopathic focal dystonia.
AOIFD presenting as musician’s dystonia mostly affects males with age of onset under 40 years. After 45 years, AOIFD phenotypes predominantly affect females and, with increasing age at onset, there is a steady decrease in the proportion of males affected.
This review of the total spectrum of the syndrome aims to holistically explain the pathophysiology of the diverse symptomatology as being caused by dysfunction within the collicular–pulvinar–amygdala network.
ADULT-ONSET IDIOPATHIC CERVICAL DYSTONIA/CERVICAL DYSTONIA
Cervical dystonia (CD) is the most common expression of AOIFD, characterised by sustained, involuntary contractions of the cervical neck muscles.5 Originally, cervical dystonia was considered a psychogenic disorder. The first book on movement disorders by Henri Meige (1901)6 described “le torticollis mental” (cervical dystonia) as a tic disorder. The peculiar “geste antagoniste” was used as evidence for its psychogenic nature. This belief persisted until the late 1900s, when a conceptual change occurred in how these conditions were viewed.
CERVICAL DYSTONIA: CLINICAL CHARACTERISTICS
Demographics
CD affects females more than males (with a ratio of 1.5 females to every 1.0 male). The mean age of onset for females is 45.9 years, and the mean age of onset for males is 5.0–6.0 years earlier, at 40.0 years.3,5,7 Delays in diagnosis complicate research into cervical dystonia, and accurate assessment of age of onset can be difficult. This disorder is poorly recognised. One Australian study found a mean delay to diagnosis of 6.8 years.8 Referral by primary care physicians to non-neurologists is also common due to symptomatic overlap with musculoskeletal disorders. Many patients are initially told their symptoms are secondary to anxiety or depression.8
Risk Factors
AOIFD is an autosomal dominant disorder with poor penetrance.9 It is likely that environmental influences trigger both penetrance and expression of these genes and development of CD, or another AOIFD phenotype. Studies have suggested various risk factors associated with the development of the different types of AOIFD.10 Neck and trunk injuries were associated with cervical dystonia in a large Italian registry.11 Surgical procedures and car accidents requiring hospital attendance have also been linked with CD.12 Although car accidents are associated with whiplash and secondary cervical dystonia, and there is a lot of overlap between the disorders, certain features can be used to distinguish this from idiopathic cervical dystonia.12,13
Motor Features
Patients with CD frequently show an irregular, arrhythmic, ‘spasmodic’ jerking movement of the head with slower recovery phases towards the neutral position. Slow rhythmic deviations, sustained tonic movements, and permanent fixed deformities can also be seen.14 Specific symptoms, pain, and social anxiety associated with these should be probed in patients for targeted treatments.15 Head tremor in cervical dystonia can be a troublesome symptom, and is often the most debilitating. It can be seen in up to 80% of CD cases and has an association with positive family history.16 Tremor associated with dystonia is a separate entity and is defined as the presence of tremor in a body part not affected by dystonia, e.g., limb tremor in patients with CD.16 Clinically, limb tremor in CD can be indistinguishable from essential tremor. Tremor amplitudes are subclinically larger in patients with essential tremor, and limb tremor in CD is often more irregular, although it can be difficult to identify this on examination.17 A list of differential diagnoses is given in Table 2.

Table 2: Cervical dystonia: differential diagnoses.14
Sensory Features
Numerous sensory changes have been associated with focal dystonias, and will not be discussed here.18 One feature, however, is an important diagnostic clue: the “geste antagoniste,” a term first introduced in Meige’s book on movement disorders.6,19 This ‘sensory trick’ can be used by patients to attenuate the symptoms of CD. In one study of 50 patients, all had at least one geste involving arm movement towards the face or head. Over half of patients have more than one geste. The cheek and chin are areas most commonly involved.20 Tactile stimuli are thought to modulate sensorimotor feedback and diminish motor output, although specific mechanisms remain elusive. This unique sign may be lost with disease progression.21
Pain is a prominent symptom and can affect the majority of patients with CD. It has been described as “continuous,” “radiating,” and “tiring” and is often unilateral.22 It can be the presenting symptom prior to onset of motor features.23 The high prevalence is unique to CD among the various focal dystonias. The presence of pain may be related to muscle hypertrophy from forced contractions or due to higher density of pain receptors in affected muscles.24 Pain is not always related to dystonia severity, and contributes significantly to reduced quality of life (QoL) in CD.25
Psychiatric Symptoms
Anxiety and/or depression are important features of AOIFD. Anxiety and depression can affect up to 40% of patients with CD, depending on the assessment method used.26 These symptoms often have a bigger impact on QoL in CD than the motor dystonic features.27 Although the gold standard assessment method would be psychiatrist-led clinical review, this is impractical in a neurologist-led botulinum toxin service. The Beck Anxiety Index (BAI)28 and Beck Depression Inventory (BDI)29 are frequently used, but are not disease-specific. These brief screening tools are easily applied, short, and self-administered. High scores, indicating excess symptoms of anxiety or depression, should prompt neurologists to further probe these symptoms.
Cognitive Symptoms
A detailed account of cognitive changes is beyond the scope of this manuscript. Clinicians treating patients with AOIFD should be cognisant that dystonia is more than a motor disorder. Various cognitive changes have been noted in the AOIFD literature, e.g., using basic screening tools to cognitive impairment,30 executive function and working memory,31 deficits in cognitive flexibility,32 and sustained attention.33 The authors’ research group has found changes in social cognition,34 although this is contested and warrants further probing.35 Research into cognitive symptoms can be difficult due to variations in AOIFD, variations within AOIFD, disease rarity, and consistency across measurement tools.
Treatment
Oral medication options for CD are limited. Generally, anticholinergic medications are most effective. A majority of patients with dystonia trialled on high dose trihexyphenidyl showed a benefit, with over one-third of patients showing a dramatic benefit over long-term use. However, this study involved younger patients with mixed dystonia aetiologies.36 Long-term outcomes may differ for AOIFD. A limiting factor in anticholinergic use are the side effects, which can be significant in older patients. Important unwanted impacts include memory loss, confusion, and insomnia in adults, and chorea or exacerbation of pre-existing tic disorders in children.37 Peripheral anticholinergic symptoms, e.g., urinary retention, can be difficult to manage. Oral pyridostigmine can mitigate some side effects. Anticholinergic drugs can also be abused, trihexyphenidyl in particular, as it can cause a perceived sense of relaxation or euphoria, and is of particular concern in patients with psychiatric comorbidities.38
Benzodiazepines, particularly clonazepam, are commonly used in focal dystonias. They may be especially effective in treating CD with head tremor. An initial dose of 0.5 mg clonazepam in the evenings can be trialled.37 Clonazepam may be more useful in patients with milder disease, and in blepharospasm.39
Botulinum toxin (BoNT) is the most effective treatment for focal dystonias. It began to be used for therapeutic purposes in the 1980s. This toxic compound is produced by Clostridium botulinum and has seven different forms (A–G); Types A and B are used for clinical treatment purposes. The toxin works presynaptically by blocking the binding of acetylcholine-containing vesicles with the cell membrane, preventing acetylcholine exocytosis. It results in muscle paresis and can cause muscle atrophy.40 In cervical dystonia, BoNT can be given without using ultrasound or electromyographic guidance; this is routine practice at the authors’ centre. Careful visual inspection and palpation is usually sufficient for a good outcome. Even though clinical improvement is seen, palpation has been shown to inferior to ultrasound in terms of accuracy of injection, in particular for splenius capitis and deeper muscles.41,42 In the authors’ experience, ultrasound can provide confidence to neurologists starting BoNT injections in avoiding vasculature and in patients with obesity. However, as with any medical device, there is a learning curve. BoNT improves dystonic posturing, pain, and QoL in patients with CD. Guidance is available for recommended BoNT dosage following muscle identification.42 It can take up to 2 weeks for the therapeutic effect to be seen, and repeat treatment is advised at 12 weeks, although flexible dosing schedules should be considered for appropriate patients.42 The most common adverse effects are muscle weakness, dysphagia, dry mouth, pain, and influenza-like symptoms. These symptoms are typically transient, and severe events are rare.43 A combination of botulinum toxin treatment and physiotherapy can be more effective than BoNT alone. One study suggested that a combined approach results in lower toxin dosing and longer duration of benefit of treatment; patients also reported lower pain and improved QoL.44 Neurologists should consider physiotherapy as a useful adjunct, particularly in patients who are relatively refractory to treatment with BoNT therapy alone.
Targeting of muscles is the most important aspect of BoNT therapy, and a strong knowledge of functional anatomy of neck muscles is required. Cervical muscles are arranged in redundant layers, and often, several muscles need to be injected to achieve satisfactory outcomes. Examination of a patient with CD should focus on defining the posturing and dystonic muscles. The collum-caput method allows for identification of specific dystonic muscles according to neck posturing. When injecting, clinicians should be cognisant that injecting non-dystonic muscles can accentuate abnormal posturing and make identification of abnormal muscles more difficult.45
Other targeted options for refractory CD include surgical options like deep brain stimulation (DBS) and neuro-ablative procedures. DBS targets for CD include the globus pallidus interna (GPi) and the subthalamic nucleus (STN). Typically, patients are selected for DBS treatment based on lack of response to BoNT, absence of cognitive/psychiatric issues, and significant impact of CD on QoL. Bilateral GPi DBS has been shown to improve symptoms by more than 50% over long-term follow-up.46 Improvement in pain and disability accompany the amelioration in motor symptoms.47 However, pallidal treatment is not without complications. There are reports of development of bradykinesia in non-dystonic limbs that can affect daily functioning. This phenomenon appears to impact patients that report the greatest benefit from pallidal DBS and can be mitigated, but not eliminated, with alterations to stimulation.48 Other serious adverse events include subcutaneous infection, lead dislodgement/breakage, and dysarthria, and worsening of dystonia responsive to reprogramming.49 One meta-analysis has shown similar efficacy in STN DBS and GPi DBS, with a lower rate of complications in STN DBS.50
Historically, thalamotomies and pallidotomies were standard surgical treatments for CD.51,52 Although ablative therapies can be effective, the reversibility and adaptability of DBS, which can be turned on and off, has led to it being the main surgical treatment method. Currently, pallidotomies are reserved for some patients when DBS fails.53 Peripheral denervation is no longer performed.
RATING SCALES AND MEASURING DYSTONIA SEVERITY
Tracking disease changes and benefit of treatment in CD can be difficult in clinic settings, especially in the first few months after starting treatment. A validated method for assessing changes in muscle posturing, pain, and functional disability is important for treatment titration. The utility of a rating scale depends on its reliability, validity, and easy application in busy services.14 There are a number of such scales available for use in patients with CD. The Cervical Dystonia Impact Scale 58 (CDIP-58) and the revised Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS-R) are instruments that are used both clinically and in research. The latter is probably too complex for routine use.54 The Tsui score is another objective method for rating dystonia severity, and is also well validated for use in CD. It evaluates amplitude and duration of sustained postures as well as abnormal shoulder posturing.55 However, clinicians should be cognisant that for all AOIFD, variabilities in disease severity due to stress, fatigue, and activity can confound assessments at any single time point.
ANATOMY OF CERVICAL DYSTONIA
Basal Ganglia
Traditionally, secondary dystonias have been associated with insults causing dysfunction within the basal ganglia. Lesions to the ventral anterior and ventrolateral nucleus of the thalamus, putamen, and globus pallidus can cause dystonia. Onset of dystonic symptoms is often delayed by months after the initial injury.56 More evidence for basal ganglia involvement comes from the co-existence of dystonia with other disorders classically associated with the basal ganglia, e.g., Parkinson’s disease and Huntington’s disease. However, many patients with dystonia show no evidence of structural basal ganglia abnormalities. Moreover, many patients with basal ganglia lesions do not manifest dystonia. Animal studies have also indicated that other regions functionally connected to the basal ganglia may be implicated.57
MRI evidence suggest that AOIFD is a ‘network disorder’ affecting multiple distinct regions in the brain.58 Functionally, the superior colliculus (SC) likely plays a central role in disease mechanism, and this has been corroborated, particularly through utilisation of temporal discrimination.59 The SC is a laminated, multi-layered structure that sits at the rostral most aspect of the brainstem. Functionally, it has been divided into two layers: a superficial layer, mainly visual and sensory receptive, and a deep layer, which projects motor outputs. At its deepest, the SC blends with the reticular formation. Ventromedially lies the periaqueductal grey matter.60
AOIFDs could be considered a disorder due to disinhibition at the level of the SC.
Animal studies have confirmed that SC disinhibition evokes dystonic posturing. Inhibition of the SNpr, resulting in loss of inhibition (disinhibition or excitation) affecting the SC, evokes a dystonic head tilt in macaques.61 A number of downstream motor (reticulospinal) pathways exit from the SC that can affect cervical neck muscles to explain the dystonic features. Stimulation of the SC in monkeys results in evoked EMG responses in obliquus capitis, rectus capitis, and splenius capitis.62
The temporal discrimination threshold (TDT) is the shortest time interval at which two sequential stimuli are perceived as asynchronous. It has been demonstrated previously that an abnormal TDT is an important state-independent endophenotype in CD.63,64 Abnormal TDTs are easily assessed clinically and are a sensitive measure of defective processing within the SC.65 The SC detects salient environmental change and neurons enter an ‘on’ state. SC cells exhibit an ‘on’, ‘pause’, and ‘off’ state in response to detection, persistence, and removal of stimuli, respectively. GABAergic gating shapes the generation of the pause and on phases and inhibition, e.g., with gabazine, leads to prolongation of both the on and off states, resulting in prolonged TDT.66 Patients and unaffected relatives with abnormal TDTs show reduced SC activity compared to controls, supporting the hypothesis that disrupted SC processing (loss of inhibition at the level of the SC) mediates abnormal temporal discrimination.59
Finally, it is important for any anatomical and functional model explaining AOIFD mechanisms to accommodate the non-motor symptoms as well as motor features. The authors have previously described the importance of the collicular–pulvinar–amygdala network as a potential explanation for the spectrum of changes in AOIFD and CD.67 Tractography has confirmed the existence of anatomical connections between the three regions.68 The collicular–pulvinar–amygdala network has a role in encoding emotion towards emotional/aversive stimuli,69 perceptual decision making,70 social cognition,71 depression, and anxiety.72,73 An understanding of anatomical functional networks underpinning CD can help lead to an holistic approach to patient care beyond focus on BoNT therapy alone.
CONCLUSION
Cervical dystonia is a rare, poorly recognised disorder that can have a big impact on patient QoL. A clear understanding of the presentation of CD and differential diagnoses are required to identify patients appropriate for BoNT therapy. Treatment with BoNT also takes practice, and knowledge of neck anatomy is important for positive outcomes. Clinicians should also familiarise themselves with ultrasound and electromyography guidance for patients that do not respond well to unguided injections. With careful muscle selection and accurate injections, CD can be treated very effectively. A holistic approach, with multidisciplinary input, including physiotherapists, occupational therapists, and psychologists, would be most effective in improving QoL.








