Abstract
Intramedullary spinal cord abscess (ISCA) is an exceptionally rare pathological condition with the potential to affect any segment of the spinal cord. The involvement of the cervical cord, however, is notably uncommon, with only 36% of the ISCA localised to this region. The authors present the case of a 67-year-old male who exhibited atypical clinical manifestations, including chronic neck and shoulder pain, and an acute onset of right sided weakness. Diagnostic imaging revealed a bi-loculated intramedullary ring enhanced lesion, suggestive of an ISCA. Initial intervention comprised of a cervical laminectomy and aspiration of the abscess. Despite this, the patient experienced progressive neurological decline involving all four limbs, necessitating a revision surgery with myelotomy. This was supplemented with an extended course of targeted antibiotic therapy guided by culture and sensitivity results. The patient subsequently demonstrated a gradual improvement in neurological function through comprehensive rehabilitation measures. In this instance, the ISCA was determined to be cryptogenic in origin. The patient’s extensive comorbid conditions, coupled with the use of immunosuppressive medications, were likely contributory factors to the pathogenesis of the abscess. Importantly, a centrally located spinal cord abscess is less prone to cause irreversible vascular compromise compared to an epidural abscess. Thus, early detection and prompt treatment are essential to mitigating significant morbidity and mortality associated with this condition.
Key Points
1. Intramedullary spinal cord abscesses (ISCA) are exceptionally rare, with only 202 cases reported since 1830. Cervical ISCAs are even less common, accounting for just 36% of ISCA cases. Documenting these rare occurrences is crucial for expanding the limited knowledge base and providing valuable data for future research and clinical reference.2. This case report details the clinical presentation, diagnostic process, treatment, and rehabilitation of a 67-year-old male with a rare cervical ISCA caused by Streptococcus intermedius. The patient had a complex medical history, including immunosuppressive therapy for granulomatosis with polyangiitis and colonisation with Mycobacterium avium.
3. Early recognition and intervention are of critical importance to prevent significant morbidity and mortality in cervical ISCA. The authors emphasise the need for comprehensive rehabilitative measures to enhance recovery and functional restoration, as well as heightened clinical vigilance and expertise in diagnosing such rare conditions, particularly in patients with atypical presentations and underlying immunosuppression. A multidisciplinary approach is essential for achieving optimal patient outcomes, and further research and documentation will be needed to improve the understanding and management of ISCA.
INTRODUCTION
The occurrence of intramedullary spinal cord abscesses (ISCA) is rare, a phenomenon attributable to a confluence of physiological and medical safeguards.1 A systematic review conducted in 2023 uncovered only 202 reported instances of this condition since its first documentation by Hart in 1830.2 These abscesses can involve any segment of the spinal cord; however, they predominantly manifest in the thoracolumbar region, with only 36% of ISCAs reported at the cervical level.2,3
The pathogenesis of these abscesses generally involves haematogenous dissemination of bacteria or pathogens from a distal infection site. This pathway is rarely associated with spinal cord involvement due to the body’s robust immune defences and the infrequency of pathogens that compromise these barriers. Factors such as immunosuppression or invasive medical procedures are critical in breaching these defences and facilitating the development of such abscesses. The integrity of the blood–brain barrier plays a pivotal role, meticulously regulating the ingress of infectious agents into the central nervous system, thereby substantially mitigating the risk of spinal cord infection.4
Complementing this, the spinal cord’s encasement within the vertebral column and protective meninges considerably diminishes its direct exposure to pathogens. Moreover, the adept management of primary infections, such as antibiotics in contemporary clinical practice, further reduces the potential for bacterial dissemination to the spinal cord. The diagnostic complexity posed by the nonspecific symptomatology of intramedullary abscesses, which frequently mimics other neurological disorders, also likely contributes to their underdiagnosis and underscores their rarity. These factors collectively elucidate why ISCAs remain a rare clinical entity, emphasising the imperative for heightened clinical vigilance and expertise in their identification and management.
This case report delineates a rare instance of a cervical ISCA caused by Streptococcus intermedius. It underscores the importance for prompt identification and intervention to mitigate the risk of significant morbidity and mortality. Additionally, the report highlights the crucial role of rehabilitative measures in enhancing recovery and functional restoration for patients afflicted by this uncommon condition.
CASE PRESENTATION
In this case report, the authors detail the clinical presentation of a 67-year-old male who presented to the emergency department with an acute onset of right-sided weakness, preceded by a protracted history of cervical discomfort and persistent bilateral shoulder pain lasting several months. The patient’s medical history is notable for seronegative granulomatosis with polyangiitis, manifesting as recurrent respiratory and renal involvement. For the past 8 years, the patient has been maintained on an immunosuppressive therapy regimen comprising cyclophosphamide, methotrexate, mycophenolate mofetil, azathioprine, and prednisolone. This regimen has been meticulously adjusted and modified by his rheumatology team to address and manage recurrent disease relapses. Additionally, the patient exhibited a confirmed colonisation with Mycobacterium avium, for which he received antituberculosis therapy including ethambutol, azithromycin, and rifampicin for a period of 2 years. Moreover, he also had a history of a right-sided cerebrovascular accident manifesting as a transient ischaemic attack, which resolved spontaneously without residual focal neurological deficits.
Upon admission, a comprehensive neurological assessment revealed motor strength graded at 2 out of 5 across the myotomes from C5 to T1 in the right upper limb, and 3 out of 5 in the myotomes from L2 to S1 in the right lower limb, according to the Medical Research Council (MRC) scale for muscle strength. Additionally, a sensory deficit was observed in the right upper limb from C5 to T1, and throughout the entire right lower limb.
The differential diagnosis for this patient’s presentation was extensive and multifaceted. While ISCA was a consideration, particularly given his immunosuppressed state and chronic M. avium infection, transverse myelitis was also strongly considered, especially in the context of his autoimmune condition, granulomatosis with polyangiitis, which typically presents with acute or subacute motor and sensory changes. The chronic cervical and shoulder pain, coupled with acute neurological symptoms, suggested the possibility of a spinal cord tumour, such as meningiomas, schwannomas, or metastatic lesions. Additionally, the presentation was indicative of potential cervical spondylotic
myelopathy, particularly considering his age and chronic cervical discomfort. Given his history of transient ischaemic attack, vascular myelopathy or a spinal cord infarction were critical differentials, as both conditions are known to present with sudden-onset weakness and pain. Furthermore, Brown–Séquard syndrome was considered due to the specific distribution of neurological deficit. However, the absence of any noted trauma made this diagnosis less likely.
Comprehensive clinical examination, imaging, and relevant laboratory investigations were essential to accurately diagnose and effectively manage this case. MRI with gadolinium contrast of the cervical spine (Figure 1A and 1B) revealed a well-defined, bi-loculated intramedullary ovoid lesion, localised to the posterior aspect of the spinal cord at the C2/C3 level on T2-weighted images. Correspondingly, T1-weighted images (Figure 1C and 1D) demonstrated a rim-enhancing lesion at the same anatomical level. The lesion consisted of a large, left sided locule measuring 23.3 x 10.1 mm and a smaller right sided locule measuring 10.5 x 10.1 mm. Based on his culture results identifying S. intermedius and its antibiotic sensitivities, including sensitivity to vancomycin and penicillin, and resistance to clindamycin, the patient was treated with ceftriaxone for a duration of 1 month. In addition, the patient underwent a C2–C4 laminectomy and abscess aspiration. Despite the initial surgical intervention, he subsequently developed weakness in all four of his limbs, accompanied by bladder and bowel incontinence.
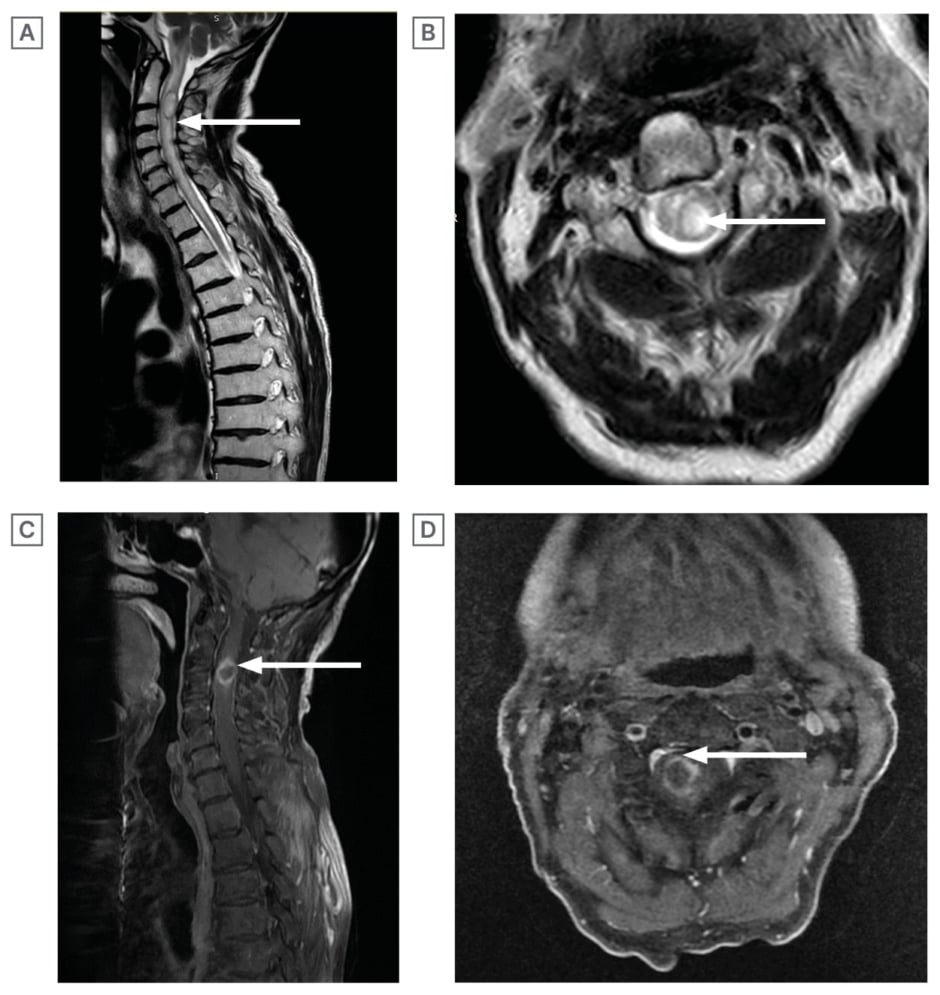
Figure 1: MRI with gadolinium contrast of the cervical spine and T1-weighted images.
A) T2-weighted MRI Cervical Spine with Gadolinium (Sagittal section): relatively well-circumscribed, biloculated intramedullary, ovoid lesion centred in the posterior aspect of the cord at the C2 and C3 level keeping with a spinal cord abscess.
B) T2-weighted MRI Cervical Spine with Gadolinium (Axial section): the lesion consists of a large left sided locule (20.3×10.1 mm) and a small right sided locule (10.5×10.1 mm).
C) T1-weighted MRI (Sagittal section): ring enhancing right sided cervical cord lesion at the C3 level. D) T1-weighted MRI (axial section): ring enhancing right sided cervical cord lesion at the C3 level
Subsequent examination revealed that muscle strength across all myotome distributions of both the left upper and lower limbs was uniformly assessed at Grade 3 out of 5 on the MRC scale. A repeat MRI scan with gadolinium on the third post-operative day demonstrated a re-accumulation of the bi-loculated abscess, measuring 22 x 18 mm, accompanied by extensive oedema both above and below the lesion (Figure 2A and 2B). Consequently, the patient underwent a revision surgical procedure, involving drainage of the intramedullary abscess via myelotomy.
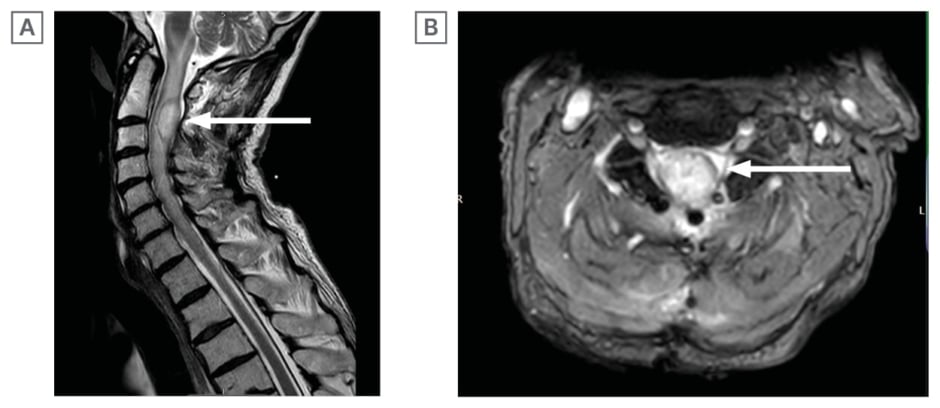
Figure 2: Repeat MRI scan with gadolinium on the third post-operative day.
A) T2-weighted MRI cervical spine with Gadolinium (Sagittal section) 3 days post laminectomy and aspiration: recollection of biloculated C2/C3 intramedullary abscess, which has increased in size (22×18 mm).
B) T2-weighted MRI cervical spine with Gadolinium scan (Axial section) 3 days post laminectomy and aspiration: extensive oedema noted at the C2 and C3 level with recollection of abscess
Over the ensuing 2–3 weeks post-myelotomy, there was a marked improvement in muscle strength across all myotomal distributions of the left upper and lower limbs, progressing to Grade 4 out of 5 on the MRC scale.
The patient’s ongoing immunosuppressive therapy and antituberculosis medication significantly elevated his risk for abscess formation throughout the body, which may also explain the absence of fever at the time of presentation. Streptococcus, the organism isolated from the intramedullary abscess, likely disseminated following transient bacteraemia from an indeterminate source. Initially, the patient exhibited asymmetric weakness predominantly affecting the right side of his body. This asymmetry correlated with the pressure effects exerted by the intramedullary abscess, likely due to the oedema distribution favouring the right side of the cervical cord, proximal to the abscess location.
Once stability was established, the patient was transferred to a specialised tertiary spinal rehabilitation centre for further rehabilitation. Upon admission, a comprehensive neurological examination classified him as C5 American Spinal Injury Association Impairment Scale (AIS) Grade D according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNSCI). Motor function assessment using the MRC scale revealed a muscle strength Grade of 2 out of 5 in all myotomes of the right upper limb, and 3 out of 5 in all myotomes of the right lower limb. The left upper and lower limbs demonstrated muscle power of 4 out of 5 on the MRC scale. Both anal tone and voluntary anal contraction were present, though weak, and deep anal pressure was also present.
Throughout the neurorehabilitation process, which employed a comprehensive multidisciplinary approach involving rehabilitation physicians, specialists, nurses, physiotherapists, occupational therapists, psychologists, dietitians, and volunteers, the patient exhibited gradual improvement. He participated in daily physiotherapy sessions lasting 1–2 hours, with gradual progressions in both gym and pool settings to enhance his mobility. Additionally, he engaged in weekly occupational therapy sessions aimed at improving dexterity and daily living activities. These interventions were designed to equip him with the necessary skills to return to his optimal state and establish a new baseline for engaging in everyday life.
During his 3-month tenure at the rehabilitation unit, he progressed from relying on an assistant-propelled wheelchair to standing unaided for brief intervals. Additionally, he advanced to taking several assisted steps and regained bowel continence. A repeat MRI scan performed 4 months after the initial presentation revealed a reduction in the lesion size to 14 x 6 mm, with features more indicative of myelitis rather than an abscess (Figure 3A–3C).
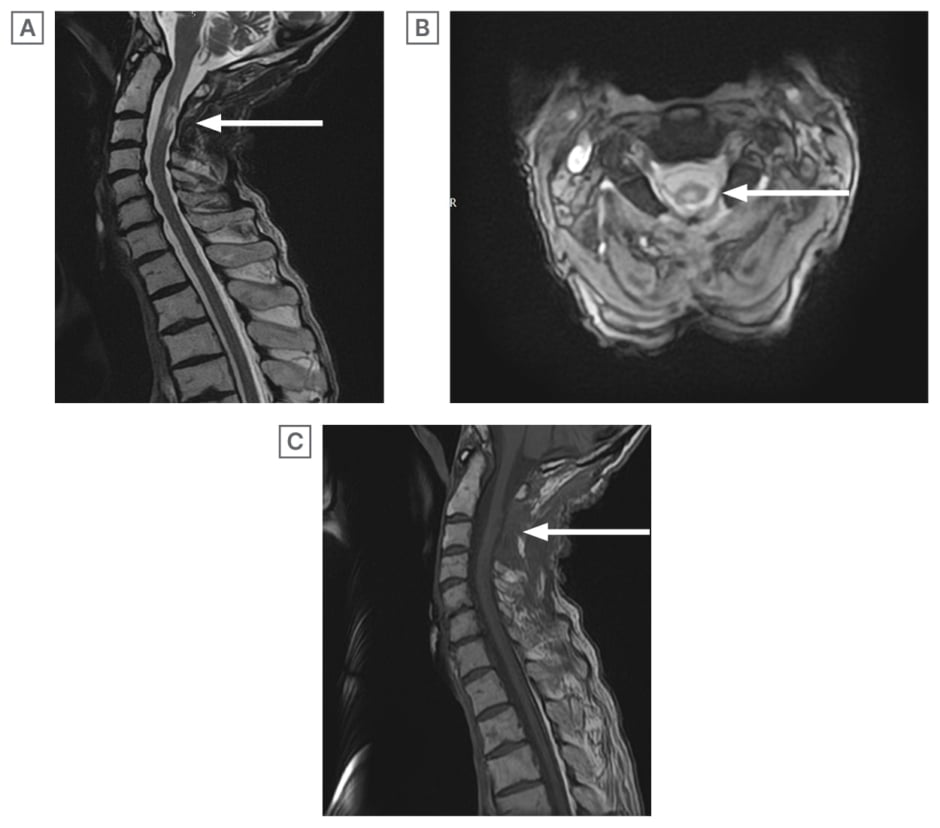
Figure 3: A repeat MRI scan performed 4 months after the initial presentation.
A) T2WI MRI cervical spine (sagittal section) 4 months post initial laminectomy and aspiration: further reduction in size of the intramedullary lesion. Features consistent with myelitis rather than abscess. Maximum diameters 14×6 mm.
B) T2WI MRI cervical spine (axial section): maximum diameter of the lesion measures 14×6 mm.
C) T1WI MRI cervical spine (sagittal section) 4 months post initial laminectomy and aspiration: absent rim enhancing lesion seen in initial T1WI.
WI: weighted image.
Although his overall neurological level remained unchanged 6 months post-admission to the rehabilitation centre, a significant improvement in the functional capabilities of his lower limbs was observed. Throughout his tenure at the rehabilitation unit, the patient demonstrated incremental progress across all domains of the Spinal Cord Independence Measure (SCIM), encompassing self-care activities (such as feeding, grooming, bathing, and dressing), respiration and sphincter management, as well as patient mobility, including transfers to and from bed, and navigating indoor and outdoor environments.2,5,6 His SCIM on admission was 26, which subsequently improved to 68 at 6 months. Upon discharge, he continued to require catheterisation, with a plan in place to transition to a supra-pubic catheter at an appropriate future date. Given the persisting weakness in his upper limb strength, he was evaluated and considered unsuitable for self-intermittent catheterisation. Additionally, he remained continent with bowels.
DISCUSSION
ISCA is an exceptionally rare condition, largely attributable to the spinal cord tissue’s inherent resistance to infection. Its infrequent documentation in the literature further limits comprehensive understanding. The exceptional nature of this case is underscored by the involvement of the cervical cord and the patient’s immunosuppressive condition; while intramedullary abscesses typically present with acute neurological deficits and initial dorsal pain followed by the onset of neurological deficits, the patient exhibited a more insidious presentation resembling a spinal tumour or other conditions causing chronic myelopathy.
ISCA predominantly affects males and has a median onset age of 45 years.1 Remarkably, 31% of cases occur in the absence of any identifiable predisposing conditions.1 The clinical manifestation of ISCA is categorised based on the duration of symptoms into acute (less than 1 week), subacute (1–6 weeks), and chronic (more than 6 weeks) phases.2 Motor and sensory impairments constitute the predominant clinical manifestations of ISCA, with most patients initially presenting with symptoms of weakness, pain, and bladder dysfunction. Furthermore, a substantial proportion of these patients are non-ambulatory at the time of initial presentation.1 Fever is observed in less than 50% of cases as an initial symptom.3 The most frequently reported symptoms at presentation include fever, pain, and neurological deficits, collectively referred to as the ISCA triad. Yet, fever is observed in less than 50% of cases as an initial symptom, and this triad is often not present in patients with subacute and chronic stages of ISCA.3 In the absence of febrile response or other definitive indicators of infection, as demonstrated in this patient, differentiating ISCA from other acute to subacute aetiologies of myelopathy, such as immunological or neoplastic processes, presents a significant diagnostic challenge. Therefore, it is imperative that clinicians maintain a high degree of suspicion for ISCA, even in the absence of classic infectious manifestations. This vigilance is critical to ensuring timely and accurate diagnosis, thereby facilitating appropriate therapeutic interventions.
The pathological evolution of an ISCA begins within the grey matter, which is rich in neuronal cell bodies, and progressively extends into the peripheral white matter, characterised by myelinated nerve fibers.4,5,7 This expansion follows a rostro-caudal trajectory, disrupting and separating the spinal cord’s fibre tracts. Notably, compression of spinal cord structures, a critical phase in the disease progression, manifests in the advanced stages of abscess development. Concurrently, the infection precipitates fibrous proliferation and gliosis around the affected area, indicative of the body’s reparative response to inflammation and injury, albeit contributing to pathological alterations within the spinal cord.
This process is further compounded by meningeal thickening, reflecting the extensive inflammatory response. In later stages, vascular complications arise, including thrombosis of adjacent veins, which compromises blood flow and exacerbates the condition’s severity.4
This suppurative condition is akin to the pyogenic brain abscess, with similar progressive changes in MRI finding in the spinal cord as one would observe in the brain in pyogenic abscesses.8,9 The lesion presents as hyperintense on T2-weighted imaging with poorly defined enhancement observed in post-contrast T1-weighted imaging initially. Additionally, in literature, post-treatment initiation, the lesion typically exhibits a reduced hyperintense appearance on T2-weighted imaging and manifests well-demarcated enhancement in post-contrast T1-weighted imaging.8,9 In majority of cases, this is not identified in earlier stages as the disease process is still under evolution. The return of function in those with intramedullary abscess is in clear contrast to those with epidural abscess,7 whereby a good functional outcome is either unknown or less, once the conduction is affected in individuals with epidural abscesses.10 Comparatively, a centrally placed abscess is less likely to cause irreversible vascular changes in the cord.
The aetiology of ISCA was identified predominantly as contiguous spread via a sinus tract opening, particularly noted in the lumbar region, though it can affect any segment of the spinal cord.5 Additional pathways for infection include haematogenous dissemination, complications following neurosurgical interventions, and cryptogenic origins.3 Specifically, cryptogenic ISCA predominantly manifests in the cervical and thoracic segments. This predilection is supported by findings that the blood supply, and correspondingly the number and calibre of blood vessels, in the upper spinal segments surpasses that of the lower segments.6 Furthermore, studies have posited that cryptogenic brain abscesses may arise from transient bacteraemia originating from an odontogenic source,2,3 suggesting a similar pathogenic mechanism for ISCA. In the era following the widespread use of antibiotics, the majority of ISCA cases have been cryptogenic, attributed to transient bacteraemia potentially leading to the colonisation of regions within the spinal cord previously compromised by subclinical injury or microinfarction.3
A recent systemic review noted there to be 202 cases of ISCA reported in literature.2 Between 1830–1944 (pre-antibiotic era), mortality rate was found to be 90%.10 The initiation of antibiotics improved the mortality rate, with subsequent mortality rates of 24% for cases reported between 1944–1977.11 In the pre-antibiotic era, over 50% of patients had haematogenous spread of infection from extraspinal focus, and more than 20% of patients had underlying suppurative lung disease. The other sites included soft tissue infections and infective endocarditis.12 Staphylococcus was found to be the most common organism followed by Streptococcus, Escherichia Coli, Proteus, Listeria, Bacteroides, Pseudomonas, Brucella, Histoplasma, Actinomyces, Candida albicans, and Mycobacterium.10,13-20 Since the advent of antibiotics, the incidence of ISCA originating from haematogenous dissemination of infection from extraspinal sources has declined to less than 10%.3 This reduction can be attributed to the broader accessibility and effectiveness of antibiotics in treating the primary infection sites. Furthermore, a study by Hoche et al.,21 involving the introduction of micro-organisms into the circulatory system of experimental animals, revealed that ISCA did not develop unless thrombi were also introduced. This led to the conclusion that vascular disruption plays a pivotal role, suggesting that thrombi might either promote the localisation of circulating pathogens within the spinal cord, or compromise its blood supply, thus fostering an environment suitable for abscess formation. Gupta et al.21 reported a case of a patient with Pott’s spine who, failing to respond to anti-tuberculous treatment, developed ISCA, necessitating surgical decompression alongside antibiotic therapy. Conversely, Kurita et al.22 reported successful treatment with antibiotics alone. They reported no difference in the neurological manifestations in those treated with surgically treated patients when compared to the medically managed patients.22 Another study highlighted a mortality rate of 13.6% among surgically treated cases.20 Historical data further illustrate that, prior to the widespread use of antibiotics (before 1929), 34 patients treated non-operatively succumbed to the condition, 31 of whom had not received antibiotic treatment, underscoring the transformative impact of antibiotic therapy on the management of ISCA.20
In the era following the widespread use of antibiotics, coupled with advancements in diagnostic and surgical techniques, surgical drainage has been recommended to prevent neurological deficits. Early detection of the condition, followed by prompt surgical intervention alongside antibiotic therapy, is regarded as the optimal treatment approach.11,19,23 If left unaddressed, the natural progression of ISCA culminates in irreversible neurological impairment due to the abscess’s capacity to occupy space, significantly contributing to the ensuing tissue damage.24,25 Consequently, it is imperative that ISCA be treated as a medical emergency, necessitating surgical decompression. Overall, there has been considerable reduction in the morbidity and mortality since the era of antibiotics, growing awareness, and prompt neurosurgical intervention.11,8,26
In the authors’ patient, the neurological status post-initial procedure, which encompassed laminectomy and aspiration, may have been compromised due to post-operative oedema and potentially incomplete abscess evacuation. Gradual neurological improvement was observed following a subsequent myelotomy, which provided further decompression of the cord abscess. The combined approach of surgical decompression, antibiotic therapy, and rehabilitation contributed significantly to the patient’s recovery, enabling independence in wheelchair mobility over extended distances, the ability to walk a few steps with assistance, and the restoration of bowel continence.
In individuals afflicted with severe spinal cord infections, the ensuing complications can precipitate profound, life-altering consequences. There is a lack of literature documenting the importance of rehabilitation, but implementing fundamental rehabilitation strategies during the pre-operative, post-operative, and home-based care phases can significantly enhance patient outcomes by improving sensory and motor functions, fostering greater independence in activities of daily living.27 This rehabilitation journey, albeit arduous and protracted, necessitates the concerted efforts of patients, their families, and the rehabilitation team. An effective rehabilitation program aims to facilitate early mobilisation, alleviate pain, augment muscle strength, prevent muscle deconditioning, ensure stabilisation and proper posture, enhance trunk mobility, and offer education to avert secondary complications like pressure ulcers and additional medical problems. Critical to this process is the continuous re-evaluation of the therapeutic regimen and the adaptation of goals to reflect the patient’s evolving needs. Paramount among these considerations is fostering patient compliance, education, and support, thereby maximising the potential for regaining independence, particularly significant in the case of the patient under study.
CONCLUSION
This case of a patient with cervical ISCA highlights several important implications for clinical practice and research. ISCA represents a rare pathology, with cervical occurrences being particularly scarce. The misleading symptoms often necessitate early detection and immediate treatment through surgical and/or antibiotic means to mitigate morbidity and mortality. The role of immunosuppressive therapy and antituberculosis treatment in predisposing to ISCA emphasises the need for careful infection monitoring in immunocompromised patients. The patient’s atypical presentation, mimicking other neurological disorders, underscores the need for heightened diagnostic vigilance, especially in the absence of typical infectious symptoms like fever. Prompt surgical intervention was critical, as evidenced by the patient’s improvement following myelotomy. The prognosis significantly depends on the timeliness of intervention; however, the importance of rehabilitation and a multidisciplinary team approach is crucial for the patient’s optimal reintegration into society. This case also contributes valuable insights to the limited literature on cervical ISCA, calling for further research into its pathophysiology, management, and outcomes. Additionally, it serves as an educational tool for clinicians, demonstrating the complexities of diagnosing and managing rare spinal cord infections. Public health strategies should include guidelines for monitoring infections in immunosuppressed individuals and preventive measures. Finally, the establishment of a registry for rare spinal infections is recommended to facilitate research and inform clinical guidelines.







