BACKGROUND AND AIMS
Spinal muscular atrophy (SMA) is an autosomal, neurodegenerative disease of the motor neurons with onset in childhood or adolescence. Nusinersen is an antisense oligonucleotide, intrathecally administered and approved for the treatment of children and adult SMA patients.1 The aim of this cross-sectional and longitudinal study was to analyse therapy-related changes in cerebrospinal fluid (CSF) and serum parameters to evaluate the efficacy and safety of the treatment.
MATERIALS AND METHODS
Nine adult patients with SMA type 2–3, with a mean age at baseline of 42.78 ± 13.95 years, were included in the study. For each intrathecal administration of 5 mL of nusinersen, 5 mL of CSF had been previously withdrawn. CSF was collected in polypropylene tubes and analysed with a simultaneous blood sample at baseline (T0), after loading dose at Day 63 (T1), after the first (T2) and second (T3) maintenance doses, at Days 180 and 300, respectively. There was one patient who voluntarily dropped-out at T3. Serum creatinine levels, CSF leukocyte count, CSF to serum glucose ratio, CSF to serum albumin ratio (Qalb), and isoelectric focussing on agarose gels with subsequent immunoblotting were performed. Neurofilament light chain (NfL), tau, phosphorylated tau, and β-amyloid 1–42 were assayed in all CSF samples, except for NfL in three patients at T3. Motor function was assessed using the Hammersmith functional motor scale–expanded (HFMSE) and revised upper limb module (RULM) scores.
Ten subjects, matched for sex and age and without neurodegenerative or inflammatory neurological diseases, underwent lumbar puncture and were assumed as controls. Comparisons were performed by Mann–Whitney test and paired t-test. Relations between clinical and serum or CSF parameters were assessed with Pearson’s correlation.
RESULTS
CSF cell count and CSF to serum glucose ratio were within the normal range in each sample. Persistent blood–brain barrier dysfunction, expressed by Qalb>6.5, was detected in three patients from T0 and in one further patient from T1. Persistent systemic oligoclonal bands (OB) were found in five patients from T0 and in three more patients during the follow-up; one patient showed intrathecal OB from T0. Serum creatinine levels were consistently much lower than normal reference values. CSF NfL, tau, phosphorylated tau, and β-amyloid 1–42 levels at T0 in patients with SMA did not differ from those in controls, without any significant longitudinal changes (Table 1). HFMSE and RULM improved significantly, only between T0 and T1 (confidence interval: 95%; p=0.032 and p=0.017, respectively).
At T0, serum creatinine values strongly correlated to HFMSE (r=0.93; p<0.001) and RULM (r=0.799; p=0.001) at T0 and at the follow-up. Neuronal biomarkers did not correlate with functional scales at each timepoint.
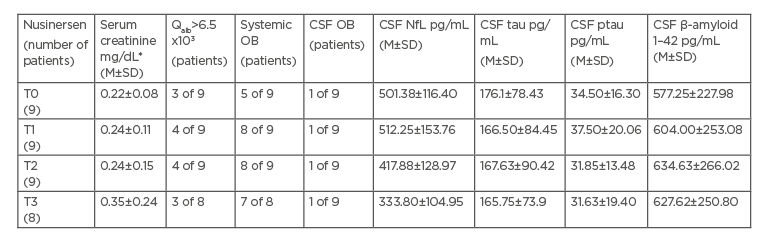
Table 1: Longitudinal serum and cerebrospinal fluid parameters in spinal muscular atrophy patients
*Serum creatinine reference range: 0.67–1.17 mg/dL
CSF: cerebrospinal fluid; M: mean; NfL: neurofilament light chain; OB: oligoclonal bands; ptau: phosphorylated tau; Qalb: cerebrospinal fluid to serum albumin ratio; SD: standard deviation; SMA: spinal muscular atrophy; T0: baseline timepoint; T1: timepoint 1, after loading dose at Day 63; T2: timepoint 2, after first maintenance dose at Day 180; T3: timepoint 3, after second maintenance dose at Day 300.
CONCLUSION
These findings of the immunological impairment in patients with SMA are consistent with other evidence of defects in multiple systems beyond motor neurons, including immune cells.2 However, the development of systemic OB during treatment could depend on changes of the immune responses induced by nusinersen against common antigens, or a drug-induced specific immune response.3 The blood–brain barrier damage may reflect a CSF flow dysfunction related to spinal stenoses of scoliosis, frequent in patients with SMA, or it may result from repeated lumbar punctures. Finally, with partial agreement with previous studies,4,5 the lack of change in the neurodegenerative biomarkers during the first year of treatment in this adult SMA cohort does not support their prognostic role.








