BACKGROUND AND AIMS
Pathology has shown that cortical abnormalities are extensive in multiple sclerosis (MS),1 whereas recent MRI studies have demonstrated that cortical damage is one of the best predictors of clinical disability and cognitive impairment in these patients.1,2 The definition of MRI measures more specifically the pathological processes affecting MS cortex might improve our understanding of the relationship between structural damage and disease clinical manifestations, thus providing novel outcome measures for monitoring MS. Neurite orientation dispersion and density imaging (NODDI) is a promising multi-compartment diffusion model to better evaluate the complexity of brain neuroanatomical microarchitecture.3 The NODDI model allows the evaluation of quantitative measures including the intracellular volume fraction (considered a measure of neurite volume), the extracellular volume fraction (representing the extracellular space), and orientation dispersion index (reflecting neurite orientation variability).3
At present, the investigation of normal-appearing grey matter abnormalities in MS using NODDI has provided conflicting and inconclusive findings.4-9 Accordingly, the application of NODDI in larger cohorts of patients with MS is necessary to provide more consistent pieces of information to better typify cortical damage in MS in vivo and to demonstrate the clinical relevance of NODDI measures.
In this study, the authors used NODDI to characterise the microstructural abnormalities of normal-appearing cortex and cortical lesions and their relations with disease phenotypes and clinical disability in a relatively large cohort of patients with MS.
MATERIALS AND METHODS
One hundred and seventy-two patients with MS (101 relapsing-remitting and 71 progressive) and 62 healthy controls underwent a brain 3T acquisition. Brain cortex and cortical lesions were segmented from 3D T1-weighted and double inversion recovery, respectively. Using NODDI, on diffusion-weighted sequence, intracellular volume fraction, extracellular volume fraction, and orientation dispersion index were assessed in normal-appearing cortex and cortical lesions.
RESULTS
The authors found that 117 out of 172 (68%) patients with MS had at least one cortical lesion. Patients with MS with normal-appearing cortex had significantly lower intracellular volume fraction versus the healthy controls’ cortex (false discovery rate [FDR]: p<0.001). Cortical lesions showed significantly increased extracellular volume fractions (FDR: p<0.001) and decreased intracellular volume fraction and orientation dispersion index versus a normal-appearing cortex of both healthy controls and patients with MS (FDR: p≤0.008). Compared with relapsing-remitting, patients with progressive MS had a significantly decreased normal-appearing cortex intracellular volume fraction and orientation dispersion index (FDR: p=0.049 and FDR: p=0.033, respectively). No cortical lesion microstructural differences were found between progressive and relapsing-remitting patients with MS. Multiple sclerosis normal-appearing cortex intracellular volume fraction, extracellular volume fractions, and orientation dispersion index were significantly correlated with disease duration, clinical disability, white matter and cortical lesion burden, and brain, grey matter, and cortical volumes (from -0.51 to 0.71; FDR: from p<0.001 to 0.049).
CONCLUSION
The authors’ study suggested that a significant neurite loss occurs in multiple sclerosis normal-appearing cortex. Cortical lesions show a further neurite density reduction, an increased extracellular space, possibly reflecting inflammation and cellular loss, and a reduced orientation dispersion index suggesting a simplification of neurites complexity. NODDI is relevant to investigate in vivo the heterogeneous pathology affecting multiple sclerosis cortex.
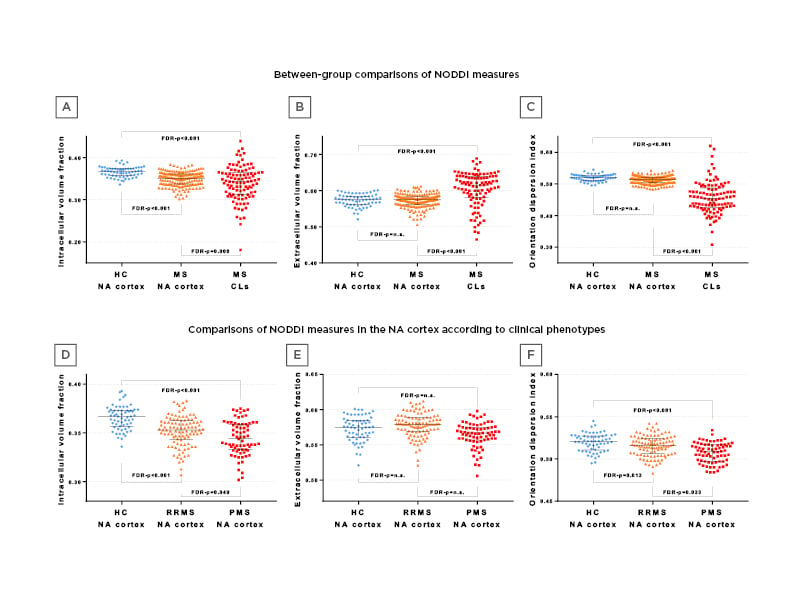
Figure 1: Comparing healthy control groups to patients with multiple sclerosis.
CL: cortical lesion; FDR: false discovery rate; HC: healthy control; MS: multiple sclerosis; NA: normal-appearing; NODDI: neurite dispersion and density imaging; PMS: progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis.








