BACKGROUND AND AIMS
Transcranial direct current stimulation (tDCS) applied over the left dorsolateral prefrontal cortex has shown to transiently improve the level of consciousness of severely brain-injured patients in disorders of consciousness,1 and seems to be effective especially for patients in minimally conscious state (MCS) compared with patients in unresponsive wakefulness syndrome.2 However, no large-sample multicentre study confirmed its efficacy or inefficacy in both subgroups of patients when applied during rehabilitation.
MATERIALS AND METHODS
In this parallel sham-controlled, double-blind, randomised trial, the authors investigated whether 4 weeks of tDCS could enhance signs of consciousness in patients in prolonged disorders of consciousness whilst in rehabilitation. TDCS (or sham) was applied at 2mA for 20 minutes over the left dorsolateral prefrontal cortex once a day, five days per week for 4 weeks, for a total of 20 sessions. The authors used the Coma Recovery Scale-Revised weekly during the treatment period (4 weeks), and at 1-, 2-, and 3-month follow-ups to objectify behavioral changes. They used a mixed general linear model to evaluate behavioral changes during the treatment period (4 weeks) and the follow-up period (3 months) between active and sham groups, accounting for diagnosis, aetiology, and time since injury. Differences (i.e., deltas) between baseline and the end of the treatment period (4 weeks), as well as baseline and the end of the follow-up period (3 months), were analysed with a Mann–Whitney test. Analyses were conducted at the group level, and by subgroup levels based on the diagnosis (MCS and unresponsive wakefulness syndrome) and the aetiology (traumatic or non-traumatic).
RESULTS
A total of 62 patients (44±14 years old; 37±24.5 weeks post-injury; 18 females [29%]; 32 MCS [52%]; 39 non-traumatic aetiology [63%]) were randomised. Thirty three patients were allocated to the active group, and 31 of them received the treatment (two dropped out right after randomisation). The remaining 29 patients were allocated to the sham group, where there was no drop out. Baseline demographic characteristics were similar between both groups. None of the patients experienced any serious adverse events related to the intervention. At the group level, the generalised linear mixed model did not reveal any treatment effect during the treatment period (p=0.21), nor during the follow-up (p=0.85). Subgroup analyses revealed a significant improvement for the active compared to the sham group for patients in MCS (p=0.020; improvement of 1 [0–3] for the active group and 0 [-2–0] for the sham group) and for traumatic brain-injured patients (p=0.021; improvement of 2 [0–3] for the active group and -2 [-4–0] for the sham group) at Month 3. No other subgroup comparisons were significant.
CONCLUSION
The authors’ results suggest that at the group level, tDCS applied during rehabilitation does not significantly enhance patients’ signs of consciousness during the treatment period. The subgroups of patients with MCS and traumatic brain injury, however, demonstrated a better recovery in the active compared to the sham group at 3-month follow-up. In this context, tDCS could be included in the rehabilitation programs of these subgroups of patients to promote their recovery.
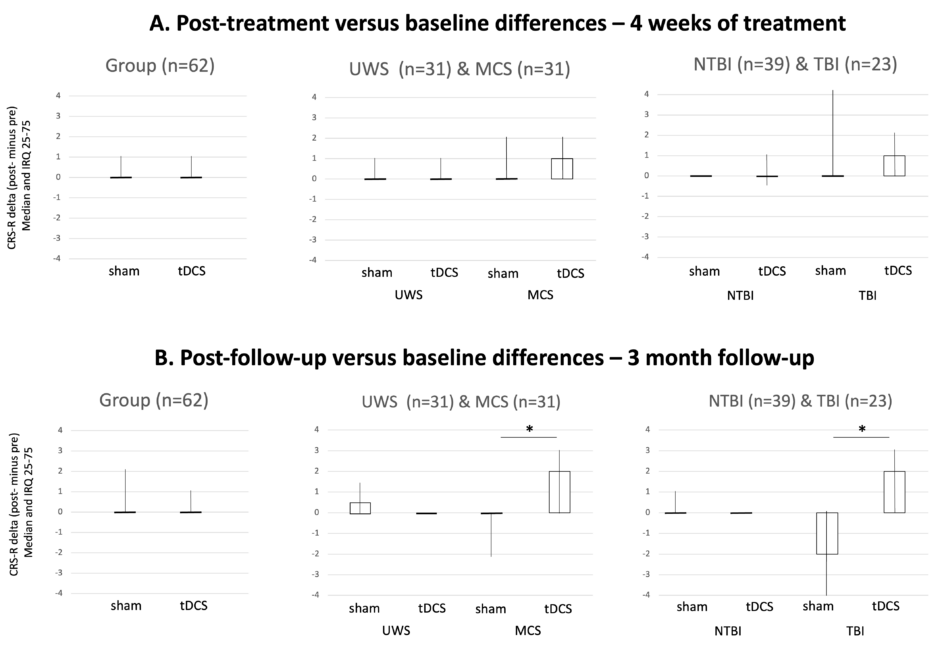
Figure 1: Results of the Mann–Whitney tests for the group and subgroups analyses.
Boxplot of active and sham tDCS at Week 4 (A. treatment period) and Month 3 (B. follow-up). Black lines represent the medians of the delta of the CRS-R total score between baseline and after active or sham tDCS; boxes represent the interquartile range; lines represent minimum and maximum.
CRS-R: Coma Recovery Scale-Revised; IQR: interquartile range; MCS: minimally conscious state; NTBI: non-traumatic brain injury; TBI: traumatic brain injury; tDCS: transcranial direct current stimulation; UWS: unresponsive wakefulness syndrome.







