INTRODUCTION
Leber hereditary optic neuropathy (LHON) is a mitochondrial disease resulting in bilateral central vision loss, primarily caused by one of three primary mitochondrial DNA (mtDNA) mutations (m.11778G>A, m.3460G>A, or m.14484T>C).1 Idebenone has shown a favourable safety and efficacy profile in the treatment of LHON.2,3 In LEROS, a Phase IV, open-label interventional study (NCT02774005), visual acuity (VA) outcomes following 24 months of idebenone treatment were compared to an external matched Natural History cohort.4 Here, the authors present the cumulative percentage of eyes with a clinically relevant recovery (CRR) from baseline (Kaplan Meier [KM] estimate) in idebenone-treated eyes by disease phase and primary mtDNA mutation, as a function of treatment duration.
MATERIALS AND METHODS
LEROS included patients with LHON aged ≥12 years and with a disease onset ≤5 years prior. Patients in the intention-to-treat cohort were treated with 900 mg/day idebenone and VA was measured over 24 months using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. CRR was defined as an improvement from ‘off-chart’ VA to at least 1.6 logMAR, or a ≥0.2 logMAR improvement if already ‘on-chart’. Eyes were stratified by time since symptom onset (subacute/dynamic [≤1 year] and chronic [>1 year]).
RESULTS
The intention-to-treat population included 196 patients with a median age of 31.9 years. The overall KM estimate of a CRR from baseline increased progressively with idebenone in subacute/dynamic eyes from 18.4% at Month 6 to 47.3% at Month 24. When assessing subacute/dynamic eyes, the KM estimate of a CRR increased progressively until the end of observation in those with a m.11778G>A mutation, from 13.0% at Month 6 to 37.7% at Month 24. The rate of CRR increased up to 12 months in eyes carrying a m.3460G>A mutation, from 14.4% at Month 6 to 33.4% at Month 12. The proportion of eyes with a m.14484T>C mutation and a CRR increased progressively from 30.7% at Month 6 to 80.0% at Month 24 (Figure 1A).5
The overall KM estimate of a CRR from baseline also increased in chronic eyes treated with idebenone, from 18.2% at Month 6 to 29.1% at Month 24. In the chronic phase, a progressive increase in CRR was observed in those with a m.11778G>A mutation, from 17.5% at Month 6 to 24.6% at Month 24. In eyes carrying a m.3460G>A mutation, the rate of CRR increased only up to 12 months from 13.2% at Month 6 to 26.0% at Month 12. In m.14484T>C eyes, an increase in CRR was observed up to Month 18, from 31.2% at Month 6 to 70.9% at Month 18 (Figure 1B).5
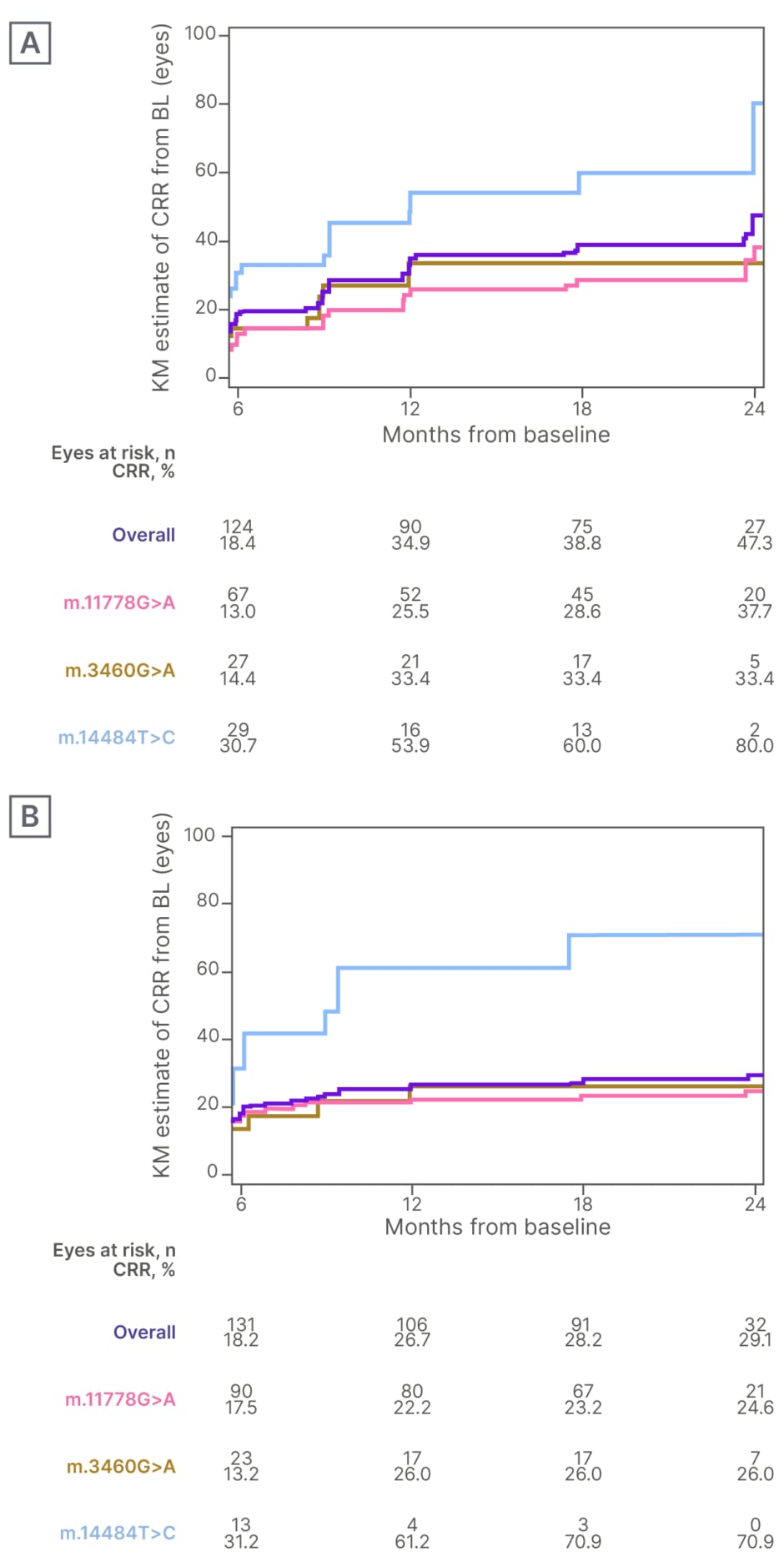
Figure 1: Kaplan-Meier estimate of a clinically relevant recovery from baseline in A) subacute/dynamic eyes and B) chronic eyes.
BL: baseline; CRR: clinically relevant recovery; KM: Kaplan-Meier.
CONCLUSION
Overall, extended duration of idebenone treatment led to an increased rate of CRR, in both subacute/dynamic and chronic eyes. The proportion of m.11778G>A eyes with CRR increased progressively until the end of observation, irrespective of disease phase. In m.3460G>A eyes, an increasing rate of CRR was only observed up to 12 months of treatment. The observed improvement was most pronounced for eyes with the m.14484T>C mutation. Conclusions for m.3460G>A and m.14484T>C eyes are limited by the number of at-risk eyes available for analysis.







