Meeting Summary
At the 56th European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Congress, held in June 2019 in Budapest, Hungary, physicians from the USA, UK, and Spain presented an educational symposium entitled ‘Survival After End-Stage Renal Failure: Preventing Cardiac Death in End-Stage Renal Disease Patients.’ During this symposium, physicians discussed concepts underlying dialysis as a chronic cardiovascular disease state; cardiovascular disease challenges with volume overload, hypertension, and heart failure; the challenge of fluid management in intermittent haemodialysis; and the effect of more frequent therapy on volume and symptom control. This review summarises the symposium.
Dialysis as a Chronic Cardiovascular Disease State
Doctor Natalie Borman
Haemodialysis was developed to alleviate symptoms due to accumulating uraemic toxins and acute fluid overload in patients with advancing chronic kidney disease and end-stage renal disease (ESRD); however, this life-saving treatment has ironically created a unique chronic disease state in dialysis-dependent patients. During the last 40 years, the leading cause of death in dialysis patients has shifted from renal failure to cardiovascular disease.1,2 This chronic disease state requires a shift in philosophy regarding the dialysis prescription. In particular, the dialysis prescription should be aimed not only at achieving adequate small solute clearance, but also by slowing the progression of cardiovascular disease and improving the patient’s tolerance of therapy.
Fluid overload significantly contributes to the development of cardiovascular disease during dialysis through several physiological pathways.1,3 First, chronic fluid overload contributes to uncontrolled hypertension, left ventricular hypertrophy, and cardiac failure.4 Second, relatively rapid ultrafiltration to remove extracellular fluid is associated with myocardial stunning, intradialytic hypotension, and increased risk of death.5 The popular thrice-weekly haemodialysis schedule includes a 2-day gap, which has been associated with increased risk of death, hospitalisation, and adverse cardiovascular events.6-8 According to new data from the USA, approximately 79% of dialysis patients have a diagnosis of diabetes, heart failure, or cardiac arrhythmia.9 An ESRD patient with any one of these conditions has an estimated 1.7–2.0 times greater risk of cardiovascular death than an ESRD patient without any of these conditions. An ESRD patient with two of these conditions has an estimated 2.5–3.6 times greater risk of cardiovascular death, and an ESRD patient with all three conditions has an estimated 5.0 times greater risk of cardiovascular death (Figure 1).9
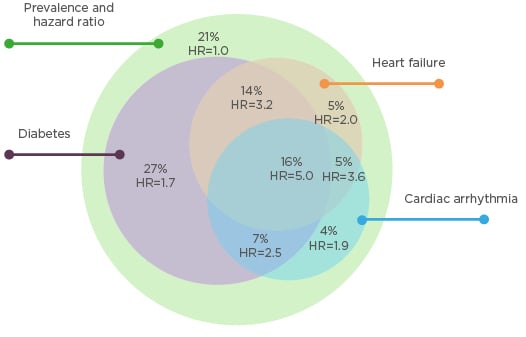
Figure 1: Stratification of cardiovascular death risk by arrhythmia, heart failure, and diabetes in a prevalent cohort of dialysis patients in the USA.9
HR: hazard ratio.
Cardiovascular Disease Challenges with Volume Overload, Hypertension, and Heart Failure
Doctor Allan Collins
Patients undergoing intermittent haemodialysis experience huge fluid shifts and often intradialytic hypotension, visible through right ventricular pressure tracing (Figure 2).10 Patients undergoing conventional haemodialysis are typically in a state of interdialytic pulmonary hypertension (right ventricular systolic blood pressure >30 mmHg). Systolic blood pressure dramatically decreases to near-normal range during dialysis treatment, but quickly returns to an elevated state during the interdialytic interval. The long interdialytic interval inherent in thrice-weekly dialysis results in patients experiencing persistent volume expansion and severe pulmonary hypertension (right ventricular systolic blood pressure >40 mmHg). This cycle of volume loading and unloading creates markedly abnormal cardiac pressure. Moreover, loading between treatments creates wall stress tension, leading to myocardial injury, cytokine production in the heart, left ventricular hypertrophy, and systolic and diastolic dysfunction.
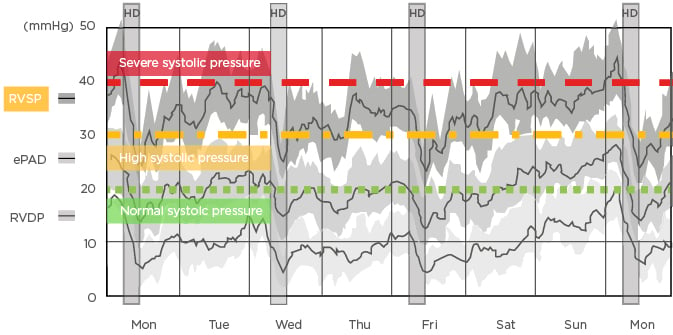
Figure 2: Changes in right ventricular pressures between haemodialysis sessions recorded by an implantable haemodynamic monitor.10
ePAD: estimated pulmonary artery diastolic pressure; HD: haemodialysis; RVDP: right ventricular diastolic pressure; RVSP: right ventricular systolic pressure.
During haemodialysis, a number of patient-related and treatment-related factors contribute to an ultrafiltration rate (UFR) that exceeds the plasma refill rate, leading to the decrease of effective arterial blood volume, the reduction of cardiac filling, the decline of cardiac output, and ultimately intradialytic hypotension. A number of interventions are recommended to alleviate intradialytic hypotension, including reduction of UFR and adjustment (or withdrawal) of antihypertensive medications.11 However, the latter of these recommendations often leads to discontinuation of cardioprotective medications, such as beta blockers and renin-angiotensin system inhibitors, which have been associated with lower risks of morbidity and mortality in patients with advanced kidney disease.12 Thus, although this action alleviates intradialytic hypotension, it removes treatments which address heart rhythm, cardiac stress, and chronic hypertension. Alternative haemodialysis regimens are needed to treat heart failure and diastolic dysfunction in the dialysis population.
Challenge of Fluid Management in Haemodialysis
Doctor Maria Fernanda Slon
Clinical practice guidelines recommend a UFR during haemodialysis that achieves volume control and minimises haemodynamic instability and intradialytic symptoms, yet the major factors influencing volume control (i.e., accurately measuring dry weight, limiting interdialytic weight gain, and minimising the fluid removal rate) are challenging to manage and are often unaddressed in dialysis patients.13 There is growing evidence that high UFR is associated with intradialytic hypotension, myocardial stunning, hypervolaemia, cardiac structural changes, and greater risk of morbidity and mortality.8,14-17 However, the optimal range of fluid removal rate is not clear.
A number of studies have attempted to evaluate the UFR threshold above which patient survival is impaired. In a study of prevalent haemodialysis patients, Flythe et al.14 demonstrated that the risk of all-cause and cardiovascular mortality began to increase at UFR >10 mL/hour/kg regardless of the status of congestive heart failure. However, Chazot et al.8 demonstrated that even a moderate UFR was associated with increased risk of death among prevalent haemodialysis patients; patients with UFR >6.8 mL/hour/kg experienced a significantly greater risk of all-cause mortality than patients with UFR <6.8 mL/hour/kg.8 In a study of incident patients, Kim et al.15 reported linear associations between UFR and both all-cause and cardiovascular mortality. Finally, a large retrospective cohort study by Assimon et al.16 confirmed a robust association between higher UFR and higher risk of death.
UFR is also related to recovery time through its direct effect on symptomatic hypotension and myocardial stunning. Moreover, a wide variety of symptoms during haemodialysis are frequently related to high UFR, including fatigue, intradialytic hypotension, cramps, and post-dialysis dizziness.18,19 These symptoms are significant not only as determinants of health-related quality of life, but also through an association between longer post-dialysis recovery time and greater risk of all-cause mortality.20
The challenge of mitigating UFR can be achieved either by reducing interdialytic weight gain through increased dialysis frequency or by extending dialysis treatment time.17 In patients with large weight gains or high UFR, clinical practice guidelines in the USA recommend more frequent or longer haemodialysis sessions in order to achieve optimal volume control and tolerance of dialysis sessions.13 The Frequent Hemodialysis Network (FHN) Daily and Nocturnal Trials demonstrated that the per-treatment incidence of intradialytic hypotension was lower with intensive haemodialysis compared to conventional haemodialysis, and that intradialytic hypotension was significantly less likely during longer haemodialysis sessions.21 Likewise, the FREEDOM study found that home haemodialysis for five or six sessions per week led to a clinically significant reduction in recovery time during 1-year follow-up.22 In general, lowering UFR through more frequent or longer dialysis sessions seems to lead to an improvement in recovery time, quality of life, and all-cause survival. Thus, there is evidence to support the contention that increasing treatment frequency, as well as cumulative treatment time, is an effective way to address volume control and tolerance of dialysis sessions and lower risk of dialysis-related morbidity and mortality.
More Frequent Therapy: The Key to Volume and Symptom Control?
Doctor Nicholas Sangala
More frequent dialysis sessions may control volume overload, cardiovascular risk, and patient-related symptoms among a diverse patient population with varying states of physiology, comorbidity, and lifestyle.1,3,4 This is increasingly important as patients with more complex comorbidity are reaching ESRD and requiring haemodialysis. In fact, the most frail and highly comorbid patients may experience the greatest improvement in symptoms with longer and more frequent therapy, as the therapy can alleviate dialysis symptoms such as cramps, lethargy, headaches, light-headedness, and prolonged recovery, and reduce medical complications such as intradialytic hypotension, interdialytic hypertension, and cardiac instability.
Patients receiving dialysis at home more than three times per week through Wessex Kidney Centre in Portsmouth, UK, regularly record patient-related symptoms on a digital platform. Over a period of 12 months, pre and post-dialysis systolic blood pressure, UFR, symptoms, and recovery time were recorded across 9,666 consecutive dialysis sessions in 79 patients. Despite an average age of 56 years (range: 21–77 years) and an average Charlson Comorbidity Index of 4.2 (range: 2.0–9.0), patients experienced intradialytic hypotension in only 2.8% of dialysis sessions, cramps in 3.4%, and headaches in 5.2%. Greater symptom severity appeared to be associated with greater haemodynamic instability, as measured by the percent reduction in systolic blood pressure during dialysis. Recovery time also appeared to have a strong relationship with haemodynamic instability; patients who recovered immediately after dialysis had the least haemodynamic instability, whereas patients who required >6 hours to recover after dialysis had the most haemodynamic instability (Figure 3). Finally, patients who reported feeling below average immediately after dialysis experienced significantly more haemodynamic instability than patients who reported feeling average or above average. Although there did not appear to be a relationship between symptom severity and UFR, dialysis sessions were almost always performed with a very low UFR (i.e., <6 mL/hour/kg).
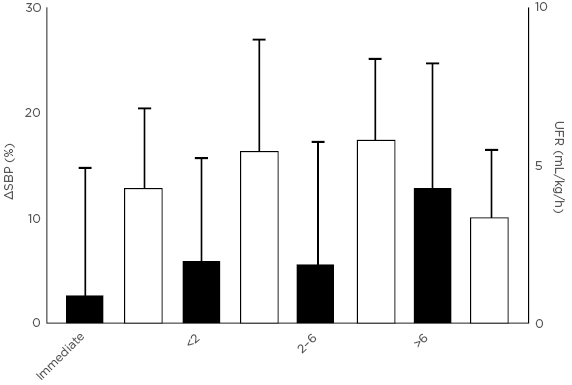
Figure 3: Individuals taking longer to recover after dialysis have poorer haemodynamic stability during dialysis (unpublished data).
SBP: systolic blood pressure; UFR: ultrafiltration rate.
The significance of haemodynamic instability on symptom control in these data support an emphasis on hydration status and the amount of fluid in the extracellular space at the start of dialysis. For example, examination of patient-level data from the Wessex Kidney Centre cohort suggests that individuals experience more severe symptoms and more haemodynamic instability with lower post-dialysis weights. Identifying an optimal target weight can help achieve asymptomatic dialysis with minimal haemodynamic instability.
UFR and hydration status are key indicators of fluid management, which can be managed effectively by altering treatment frequency. Increasing haemodialysis frequency can help improve stability, improve blood pressure control, reduce symptoms, meet individual ultrafiltration goals, allow for the use of cardioprotective medications, eliminate fluid overload resulting from a 2-day gap in treatment, and break the volume overload cycle. In doing so, patients may experience lower risk of cardiac death during long-term dialysis.








