Meeting Summary
IgA nephropathy (IgAN) is a rare, life-limiting disease for which there is significant unmet need. Until recently, drug development for IgAN had been impeded by the requirement for large-scale, long-term clinical trials to measure clinical outcomes. However, the recent establishment of ‘reduction in proteinuria’ as a surrogate endpoint to predict clinical outcomes in IgAN, as a basis for accelerated drug approval, has transformed the field. At the 60th European Renal Association (ERA) Congress in June 2023, two oral poster presentations focused on the use of early reduction in proteinuria as an endpoint for clinical studies in IgAN. Alex Mercer, Consultant in Clinical Drug Development at JAMCO Pharma Consulting in Stockholm, Sweden, presented data estimating the long-term clinical outcome of reductions in proteinuria (clinically meaningful extensions in time to kidney failure or death), which could help predict the future protective effect of any new intervention on kidney function. Following this, Jonathan Barratt, Mayer Professor of Renal Medicine at the University of Leicester, and Honorary Consultant Nephrologist at Leicester General Hospital, UK, described findings from the prespecified interim analysis of the Phase III PROTECT study of sparsentan (a novel dual endothelin angiotensin receptor antagonist) in IgAN, which included reduction in proteinuria as a primary endpoint. In patients with IgAN at high risk of disease progression, sparsentan produced a rapid and significant reduction in proteinuria of a level that, according to the study data presented by Mercer, would correspond to a substantially lowered risk of kidney failure or death. Long-term data to confirm this predicted clinical outcome on disease progression are anticipated.
Introduction
IgAN is a progressive, immune complex-mediated glomerulonephritis that is the most common form of primary glomerulonephritis worldwide.1,2 It is a leading cause of chronic kidney disease and kidney failure.1,3 The prognosis is poor, with a median length of kidney survival estimated at 11.4 years in the UK National Registry of Rare Kidney Diseases (RaDaR), and almost all patients at risk of progression to kidney failure within their lifetime.3 Indeed, as most patients with IgAN are diagnosed between 30–40 years of age, the impact of this life-limiting disease is often realised in the mid-adult years.3-6 Overall, IgAN is a disease with a high level of unmet need.
Focusing on addressing this unmet need, Alex Mercer and Jonathan Barratt presented evidence on the use of proteinuria as a surrogate endpoint for disease progression in IgAN at the ERA Congress, including an interim analysis of the PROTECT study of the dual endothelin angiotensin receptor antagonist, sparsentan, in patients with IgAN.7,8
Estimating Delay in Time to Kidney Failure or Death for Treatment Effects on Proteinuria in IgA Nephropathy
Alex Mercer
In their oral presentation,9 Mercer introduced IgAN as, “a disease in need of new medicines [within] a golden age of drug development.” Traditionally, approval of a new drug for chronic kidney disease has required treatment effect to be proven on the composite endpoint of
first occurrence of reduction in estimated glomerular filtration rate (eGFR; eGFR: <15 mL/min/1.73 m2; chronic kidney disease Stage 5), doubling of serum creatinine, and kidney replacement therapy, which has required large-scale, lengthy, randomised controlled trials (RCT). This ‘high bar’ has proven extremely prohibitive to drug development for rare kidney diseases such as IgAN. However, in a transformative step, meta-regression ‘trial level analyses’, published in 2016 and 2019, established that an early treatment effect on proteinuria predicts a treatment effect on clinical outcomes in IgAN.10,11
Mercer identified that the acceptance by the USA and European regulatory authorities of ‘change in proteinuria over 9 months’ as a surrogate endpoint for accelerated/conditional drug approval10,11 (with full approval reliant on confirmatory clinical data), has enabled studies with smaller sample sizes and an earlier readout. This advance has contributed to the expansion of the IgAN development programme from just one Phase II study in 2013 to 21 studies (Phase II/III) of new medicines in 2023. Indeed, all industry-sponsored Phase III RCTs in IgAN now have a prespecified proteinuria endpoint at approximately 9 months.
Mercer presented findings from a two-step approach that estimated the delay in time to kidney failure or death associated with treatment effects of 40% and 50% reduction in proteinuria at 9 months to allow the estimation of future benefits in ongoing Phase III studies of IgAN.9 In step one, published findings of a trial-level analysis from 13 RCTs in IgAN (n=1,153)10 were used to predict the risk (hazard ratio [HR]) that the treatment effect on proteinuria would lead to a doubling of serum creatinine, kidney failure, or death. In step two, the HRs were used to estimate the delay to kidney failure or death by applying Weibull accelerated failure time modelling to a Phase III representative IgAN population with long follow-up, the UK RaDaR IgAN cohort of adult patients (n=535), of whom 171 had progressed to kidney failure or death. Patients in the cohort were required to have an eGFR ≥30 mL/min/1.73 m2, and proteinuria equivalent to approximately 1 g/day (urine protein-creatinine ratio [UPCR]: ≥100 mg/mmol).
In step one, 40% and 50% reductions in proteinuria were associated with substantially lowered risks of kidney failure or death, with predicted HRs of 0.41 (95% confidence interval [CI]: 0.26–0.65) and 0.33 (95% CI: 0.16–0.67), respectively. In step two, the predicted HRs for 40% and 50% reductions in proteinuria were applied to the RaDaR IgAN cohort, which had a median UPCR of 1.5 g/g and a mean eGFR of 61 mL/min/1.73 m2) at baseline, with a median time to kidney failure or death of 8.6 (95% CI: 7.8–9.5) years and a 5-year survival rate of 75%. A 40% reduction in proteinuria (HR: 0.41) prolonged the median time to kidney failure or death to 14.9 (95% CI: 13.6–16.4) years (an increase of 6.3 years) with a 5-year survival rate of 89% (Figure 1). A 50% reduction in proteinuria (HR: 0.33) prolonged the median time to kidney failure or death to 17.1 (95% CI: 15.6–18.8) years (an increase of 8.5 years) with a 5-year survival rate of 91% (Figure 1).
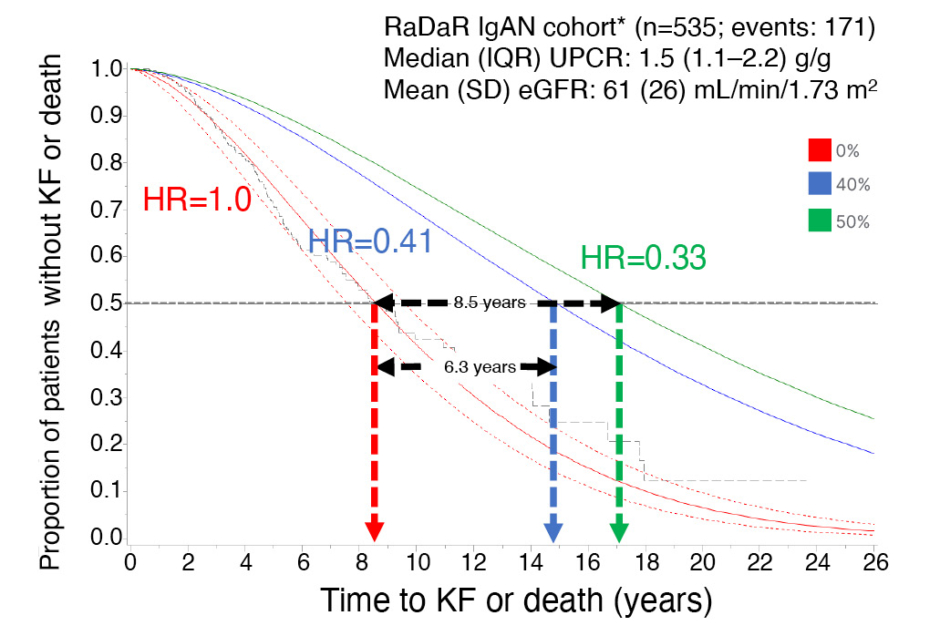
Figure 1: Estimating time to kidney failure or death for predicted hazard ratios associated with treatment effects of 0%, 40%, and 50% reduction in proteinuria.
*Representative Phase III population
eGFR: estimated glomerular filtration rate; HR: hazard ratio; IgAN: IgA nephropathy; IQR: interquartile range; KF: kidney failure; RaDaR: UK National Registry of Rare Kidney Diseases; SD: standard deviation; UPCR: urine protein-creatinine ratio.
Thus, Mercer identified that achieving 40% and 50% reductions in proteinuria at 9 months was predicted to associate with a substantially lowered risk of kidney failure or death, an increased median time to kidney failure or death, and an increased 5-year survival probability.
Mercer concluded that “therapeutic interventions that reduce proteinuria and risk of kidney failure can confer important and clinically meaningful extensions in the time patients are alive and free from kidney failure.” Mercer also highlighted that, provided baseline characteristics are comparable, their study findings would allow estimation of future benefit in delay to disease progression for proteinuria results generated in ongoing Phase III RCTs.
Superior Proteinuria Reduction with Sparsentan in IgA Nephropathy: PROTECT Study Interim Analysis
Jonathan Barratt
The PROTECT study of sparsentan in IgAN is one such ongoing, global, Phase III RCT that evaluated early change in proteinuria as a prespecified interim analysis endpoint.7,8,12 The findings of this interim analysis were presented by Barratt.13
Sparsentan is an oral, non-immunosuppressive, single-molecule dual antagonist that is highly selective for both endothelin (ET-1) and angiotensin II (Ang II) receptors.14-16 Barratt explained that inflammation and damage of the glomeruli caused by deposition of IgA-immune complexes in the mesangium triggers an increase in production of ET-1 and Ang II.17-19 Acting via their receptors (ETA and Ang II receptor Type 1), ET-1 and Ang II work in tandem to amplify this damage, impairing filtration processes and leading to the leakage of blood and protein into the urine.15,20,21 Increasing proteinuria further amplifies ET-1 and Ang II, exacerbating this cycle of damage, and driving progression towards kidney failure.15,20-22 Thus, proteinuria has a central role in IgAN disease pathology and, as described above, has been established as a surrogate endpoint for treatment effects on disease progression.9-11 Sparsentan received accelerated approval from the U.S. Food and Drug Administration (FDA) in February 2023 for the reduction of proteinuria in adults with primary IgAN at risk of rapid disease progression (UPCR: ≥1.5 g/g), with continued approval contingent upon demonstration that sparsentan slows the rate of decline in kidney function (eGFR) in the long term.16,23
PROTECT enrolled 404 adult patients with IgAN and persistent proteinuria (≥1 g/day) despite receiving maximised treatment with an angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker, who were at high risk of disease progression.7,8 Patients were randomised to receive sparsentan 400 mg once daily or current standard-of-care irbesartan 300 mg once daily, for a 2-year (110-week) double-blind treatment period.7,24 At baseline, patients had a mean age of approximately 46 years, persistent proteinuria (median 1.8 g/day urinary protein excretion; median UPCR: 1.2–1.3 g/g), and existing signs of kidney damage (mean eGFR: 57 mL/min/1.73 m2), characteristics comparable to those of the UK IgAN RaDaR cohort presented by Mercer et al.9 As reported by Barratt, a prespecified interim analysis, with primary endpoint of change from baseline to Week 36 in UPCR, was triggered 36 weeks (approximately 9 months) after the 280th patient was randomised. The longer term confirmatory composite secondary endpoint (descriptive only at the interim analysis) was the proportion of patients reaching 40% reduction in eGFR, kidney failure, or all-cause mortality.
Interim analysis showed a marked difference between the treatment groups in proteinuria reduction at Week 36, with sparsentan treatment producing a 41% relative reduction versus irbesartan (p<0.0001; Figure 2). According to the findings of Mercer et al.,9 a 40% reduction in proteinuria would predict a 6.3-year extension in time to kidney/failure death. Moreover, subgroup analysis showed that this treatment effect was irrespective of baseline factors, such as age, sex, eGFR, and level of proteinuria. Examining data available to Week 94, Barratt indicated that the difference in proteinuria reduction appeared as early as Week 4, and was maintained while patients remained on therapy (Figure 2).
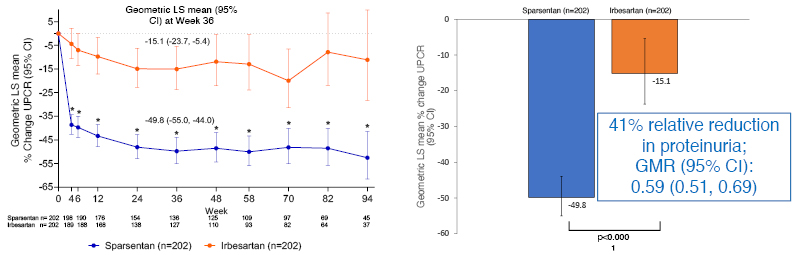
Figure 2: Percent change from baseline in urine protein-creatinine ratio (24-hour urine samples) by visit at the prespecified interim analysis of the PROTECT study.
Primary analysis set: 94% of patients treated with sparsentan and 96% of patients treated with irbesartan reached target dose.
*p<0.0001 sparsentan versus irbesartan at each week.
CI: confidence interval; GMR: geometric least squares mean ratio; LS: least squares; UPCR: urine protein-creatinine ratio.
This observation was consistent with the time to first complete proteinuria remission (<0.3 g/day), which was also significantly in favour of sparsentan over irbesartan (p=0.0002) at the interim analysis, as was the time to first partial proteinuria remission (<1.0 g/day; p<0.0001). Notably, remission events continued to occur throughout the duration of the study, with Barratt highlighting the importance of a benefit that can be accrued throughout the treatment cycle, even when no effect is observed within the early weeks.
Data from the 2-year confirmatory eGFR endpoint analyses and the secondary clinical outcome endpoint of 40% reduction in eGFR, kidney failure, or death, will be available at final analysis once all patients have completed the double-blind period. However, at interim analysis, Barratt reported that 20 patients had reached the composite clinical outcome endpoint, with a numerical difference already apparent between the sparsentan (n=7) and irbesartan (n=13) groups. It was noted that with longer follow-up, this endpoint may deliver valuable information on the link between change in proteinuria and long-term clinical outcomes on disease progression.
In terms of safety (Figure 3), PROTECT raised no concerns at the interim analysis and, overall, there were fewer treatment discontinuations of sparsentan (11%) than irbesartan (19%). Barratt noted that while there were numerically more episodes of peripheral oedema with sparsentan than with irbesartan, there were no cases of heart failure, oedema-related treatment discontinuations, or fluid retention serious adverse events. Moreover, the treatment groups were comparable in terms of new use of diuretics/loop diuretics.
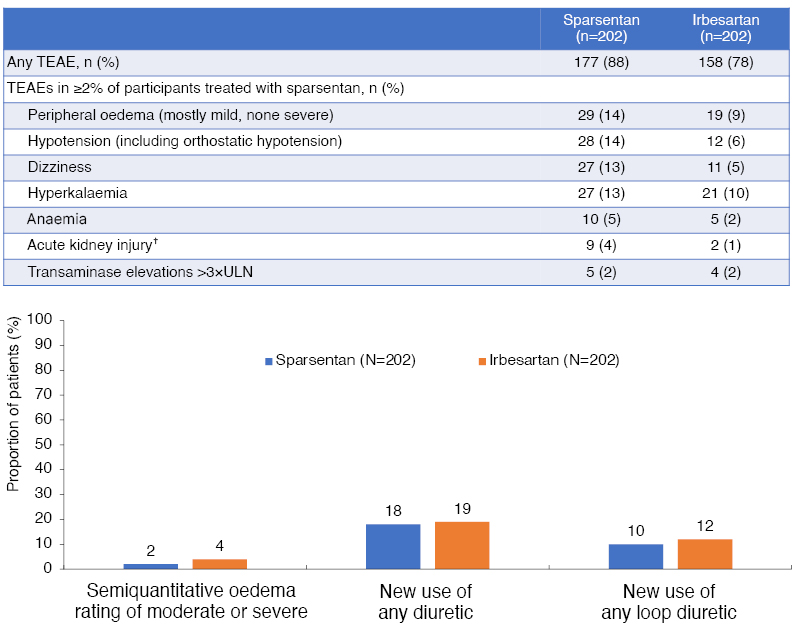
Figure 3: Treatment-emergent adverse events with sparsentan and irbesartan at the prespecified interim analysis of the PROTECT study.*
*Median treatment duration: 86.9 weeks.
†Acute kidney injury was typically reported based on changes in serum creatinine between study visits.
TEAE: treatment-emergent adverse event; ULN: upper limit of normal.
Hypotension was numerically more frequent with sparsentan than irbesartan, but blood pressure control over the 36 weeks was generally similar in the two groups. The incidence of hyperkalaemia was also comparable between groups, with no hyperkalaemia-related discontinuations. There were few instances of elevated liver enzymes (Figure 3), with all cases being asymptomatic, reversible, and having no concurrent elevation in total bilirubin.
In summary, in a prespecified interim analysis of the PROTECT study, sparsentan produced a significant and clinically meaningful reduction in proteinuria in adults with IgAN and a high risk of progressive kidney disease. The treatment effect was rapid, sustained, and superior to that provided by irbesartan, which is widely regarded as the current standard-of-care therapy. Sparsentan was also well tolerated. With the PROTECT study ongoing, Barratt said that full 2-year data output on the confirmatory eGFR endpoint is eagerly anticipated later in 2023.
Conclusions
IgAN is a disease with great unmet need for new, effective, and well-tolerated therapies. Proteinuria has been established as a surrogate endpoint for clinical outcomes in IgAN, facilitating clinical study towards accelerated drug approval. Modelling of clinical outcomes based on the IgAN RaDaR cohort have allowed the estimation of future benefits from proteinuria endpoints in Phase III studies in IgAN. In the PROTECT study, sparsentan produced an early, sustained reduction in proteinuria at 9 months that was superior to that of irbesartan, thus predicting a clinically meaningful effect on disease progression that is to be confirmed in long-term analyses.







