Meeting Summary
This symposium took place during the 60th European Renal Association (ERA) Congress, held in Milan, Italy, and virtually. Bengt Fellström, Uppsala University, Sweden, described the relationship between IgA nephropathy (IgAN) and gastrointestinal mucosal reactivity. Fellström then outlined the history of Nefecon (Calliditas Therapeutics, Stockholm, Sweden, and STADA Arzneimittel, Bad Vilbel, Germany), which was developed based on the assumption that the gut plays a major role in the pathophysiology of the disease, and that there was a high unmet need for a well-tolerated and effective therapy. Nefecon was specifically designed to target the origins of IgAN. A Phase IIb clinical trial showed, for the first time, that 9 months of treatment with Nefecon was well-tolerated and effective in patients at risk of disease progression. Jonathan Barratt, University of Leicester, UK, and John Walls Renal Unit, Leicester General Hospital, UK, presented biomarker data supporting the efficacy data in clinical trials, and presented topline data from Part B of the Phase III NefIgArd trial. Specifically, the results demonstrated an average 5.05 mL/min/1.73 m2 estimated glomerular filtration rate (eGFR) treatment benefit in favour of Nefecon versus placebo over 2 years. This confirmed that the eGFR benefit of 9 months of active treatment with Nefecon was maintained during the observational follow-up. The eGFR benefit with Nefecon versus placebo was consistent regardless of baseline urine protein-creatinine ratio (UPCR). At 2 years, the 30% reduction in UPCR in the Nefecon versus placebo arm was similar to the percentage reduction at the end of the 9-month treatment period, plus 15 months follow-up off treatment. Patients treated with Nefecon experienced decreasing levels of proteinuria while on active treatment and for 3 months afterwards, suggesting a continued biologic effect. Barratt presented UK registry data showing that, despite being treated with the current standard of care for IgAN, three-quarters of adults and half of paediatric patients developed kidney failure or died within 20 years of disease onset. Barratt suggested a paradigm shift in the treatment approach for all patients with IgAN, who have a risk of developing kidney failure in their lifetime.
Nefecon: Rationale, Efficacy, and Safety in the Treatment of Primary IgA Nephropathy
Bengt Fellström
Pathophysiology of IgAN
The pathophysiology of IgAN has been described using the 4-hit hypothesis, where galactose-deficient IgA1 (Gd-IgA1) is formed (hit 1), thereby stimulating the production of anti-Gd-IgA1 autoantibodies (hit 2).1,2 These combine to form Gd-IgA1 immune complexes (hit 3), which deposit in the mesangial area of the kidney, leading to glomerular injury (hit 4).
Associations of IgA Nephropathy with Gastrointestinal Mucosal Reactivity
Many associations have been demonstrated relating the development of IgAN with gastrointestinal mucosal reactivity, primarily involving B and T cells in the Peyer’s patches.3 Gastrointestinal inflammatory disease (Crohn’s disease and ulcerative colitis), characterised by impaired function of the mucosal barrier, is associated with an increased risk of IgAN. For example, patients with coeliac disease have a three-fold increased risk of IgAN.4 Previous studies have identified key susceptibility genetic loci encoding proteins involved in the immune defence against gastrointestinal mucosal pathogens.5
It has also been demonstrated in patients and animal models that there is an abnormal and hyper-reactive immune response to dietary components, including gluten, casein, and soy protein, in the gut mucosa in IgAN.6-8 It has also been suggested that a defective immune tolerance in patients with IgAN might lead to an abnormal response to microbiota, triggering mucosal-associated lymphoid tissue activation and subclinical intestinal inflammation.8 The mucosal-associated lymphoid tissue is rich in plasma cells that form antibodies, and it is thought that this is where the Gd-IgA1 molecules are formed.8
Nefecon Was Designed to Specifically Target the Origins of IgA Nephropathy
Based upon these findings, and a high unmet need for a well-tolerated and effective treatment, Fellström and colleagues set out to find a compound with a mode of action aimed at specifically targeting mucosal IgA synthesis in the gut. Following release in the ileum, the drug would have a high first-pass metabolism to minimise systemic exposure and side effects.9
The active substance budesonide is a highly potent corticosteroid that is absorbed in the ileum and undergoes first-pass metabolism.10 Budesonide is 90% cleared in first-pass metabolism by the liver, thereby minimising systemic side effects. It acts on the formation of Gd-IgA1 (hit 1) by reducing mucosal B cell activity and reducing the production of aberrant IgA1 compounds (i.e., Gd-IgA1), which ultimately leads to reduced mesangial and glomerular damage.11 Studies have demonstrated that Nefecon has an acceptable safety profile, with predominantly mild to moderate adverse events (AE) that were reversible upon discontinuation.9
Several trials have been conducted using Nefecon, including the Phase IIa pilot study, the NEFIGAN Phase IIb study, and the NEFIGARD Phase IIIa/IIIb trial. A Phase III open-label extension study is ongoing, which will show what effect a second treatment session may have.
The Phase II trial Showed the Safety and Efficacy of Nefecon
The Phase IIa study was an exploratory, open-label, uncontrolled study to evaluate the safety and efficacy of Nefecon.12 It enrolled 16 patients with IgAN and albuminuria who were on stable renin-angiotensin system (RAS) blockade at three Swedish centres. Patients were treated for 6 months with Nefecon 8 mg/day, and followed up for 3 months. The inclusion criteria were age ≥18 years, biopsy-verified IgAN, proteinuria (urinary albumin >500 mg/24 hours), and serum creatinine <200 μmol/L. The study showed a maximal median reduction in albuminuria of 40% at Month 8, and an improved eGFR, which increased by 8% at Month 6 (p=0.003).
This was followed by NEFIGAN, a randomised, double-blind, placebo-controlled Phase IIb trial examining the efficacy and safety of two doses of Nefecon versus placebo in patients with IgAN at risk of kidney failure. The trial enrolled 150 patients from 62 sites in 10 European countries.9 The trial design incorporated a 6-month run-in phase, in which RAS blockade was optimised to the maximum allowed or maximum tolerated dose. This was followed by a 9-month treatment phase in which patients were randomised to Nefecon 16 mg/day, Nefecon 8 mg/day, or placebo. The 3-month follow-up phase included a 2-week tapering of the drug to Nefecon 8 mg/day in those randomised to a treatment dose of 16 mg/day, and a 2-week tapering to placebo in the remaining two groups.
Patient demographics and baseline characteristics were well-balanced across the three treatment arms. The trial’s primary objective of UPCR reduction at 9 months in all treated patients was met, with a 24.4% reduction in the Nefecon groups versus a 2.7% increase in the placebo group (p=0.007).9 When the individual doses of Nefecon were examined separately, the reduction in UPCR at 9 months compared with placebo was greater in the group receiving 16 mg/day (-27.3%; p=0.009) compared with those receiving 8 mg/day (-21.5%; p=0.029). The effect of Nefecon was sustained throughout follow up.
The other important finding of the trial was that Nefecon stabilised eGFR during the 9-month course of treatment, while patients treated with placebo experienced a decline in renal function by -9.8%.9 The study also examined the effects of Nefecon on eGFR according to baseline UPCR (Fellström et al., data on file). Active treatment stabilised eGFR independent of UPCR levels, whereas in the placebo group, higher proteinuria was associated with a stronger decrease in eGFR.
Regarding treatment-emergent AEs, there were no significant changes in body weight, blood pressure, or HbA1c versus placebo, and no serious infections. The most common AEs in the total patient population, including the placebo group, were nasopharyngitis, acne, and swelling of the ankles. A total of 13 serious AEs were reported, of which four were considered to be related to the study drug. Two occurred in the Nefecon 16 mg/day arm; these were deep vein thrombosis and deteriorating renal function. Two occurred in the placebo arm; these were increasing proteinuria and deteriorating renal function. Analysis of glucocorticosteroid AEs showed that the incidence of events was as expected, with an increased level of mild-to-moderate glucocorticosteroid AEs in the Nefecon 16 mg/day group, relative to the other two arms in the study. In summary, the analysis of treatment-emergent AEs and treatment-emergent serious AEs showed no deaths and no serious or fatal infections. There were light-to-moderate glucocorticosteroid-like side effects in a small number of patients. There was no occurrence of de novo diabetes nor any increased blood pressure, increase in body weight, osteoporosis, or fractures. The Phase II clinical trial showed, for the first time, that 9 months treatment with Nefecon is well-tolerated and effective in patients with IgAN and at risk of disease progression.
Exploratory Studies Support the Hypothesis that Nefecon Targets the Origins of IgA Nephropathy
Following the NEFIGAN trial, exploratory studies have been performed, showing that 9 months of treatment with Nefecon 16 mg/day resulted in significant changes in several markers of gut-associated lymphoid tissue activity, including mucosal IgA responses to dietary antigens, serum secretory IgA levels, elements of the complement cascade, and chemokines involved in mucosal immune cell trafficking (Wimbury D et al., unpublished data). This was associated with significant reductions in serum levels of Gd-IgA1 and IgA/IgG immune complexes. These results support the hypothesis that Nefecon regulates mucosal immune dysfunction by targeting the gut-associated lymphoid tissue, and exerting a direct disease-modifying effect in IgAN. Further analyses will be performed to better understand the importance of the gut–kidney axis in the development of IgAN.
Considering the 4-hit pathogenesis model of IgAN, the clinical trial results indicate that Nefecon has an effect on hit 1, translating into reduced mesangial and glomerular damage.1,2 To summarise, there is clinical and experimental evidence of a dysregulated mucosal immune system, particularly within the gut, in IgAN. Genetic aberrations related to human leukocyte antigen, complement, and gastrointestinal inflammatory regulation are enriched in IgAN. The gastrointestinal formation of Gd-IgA1 and subsequent formation of IgG-Gd-IgA1 immune complexes in IgAN was reduced with Nefecon treatment. The NEFIGAN study showed a significant reduction of proteinuria, which translated into stabilisation of eGFR, irrespective of the degree of baseline proteinuria. The early reduction of proteinuria is a useful surrogate endpoint in IgAN, predicting reduced risk of kidney failure, as demonstrated in a meta-analysis.13 Only mild-to-moderate side effects were observed with Nefecon treatment.
Conclusion
In conclusion, the beneficial results of Nefecon treatment, which targets the mucosal immune system in the distal ileum, support the hypothesis of upstream involvement of gut mucosal immune dysregulation in the development of IgAN. The NEFIGAN trial confirms the validity of treating IgAN by targeting the mucosal immune system in the distal ileum, with a unique formulation depositing a majority of the active drug locally in the gut and minimising systemic corticosteroid exposure.
NefIgArd and Beyond: Treating IgA Nephropathy in 2023
Jonathan Barratt
Exploratory Biomarker Analyses Confirm that Nefecon Targets the Origins of IgA Nephropathy
The Phase IIb NEFIGAN trial was followed by a range of exploratory biomarker analyses to examine the mechanism of action of Nefecon, and to confirm whether it interferes with mucosal immunity (Wimbury D et al., unpublished data). These analyses used serum, plasma, and urine samples collected at baseline, 9 months, and 12 months during the trial. More than 100 biomarkers were investigated, including chemokines, cytokines (e.g., B cell activating factor/a proliferation-inducing ligand [APRIL]), complement, and urinary markers of tissue remodelling.
Taken together, the biomarker analyses from the NEFIGAN Phase II study showed a disease-modifying effect of Nefecon in IgAN (Wimbury D et al., unpublished data). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways significantly modified by treatment with Nefecon are those related to the intestinal immune network for IgA production. This finding is in line with genome-wide association studies examining risk alleles. A clear pattern emerges of a mode of action that is unique to Nefecon, targeting the mucosal-associated lymphoid tissue within the gut, and mapping to current understanding of the pathogenesis of IgAN. As discussed previously, there were dose-dependent reductions in IgA/IgG immune complexes and Gd-IgA1.14 These biomarker data support the observed efficacy in clinical trials.
Design of the NefIgArd Phase III Trial
The full results of the Phase III NefIgArd trial were presented for the first time at the 60th ERA Congress, verifying the long-term renal benefit of Nefecon over 2 years.15 The biomarker analyses performed on the Phase II population are now being repeated in the Phase III population to validate the initial findings.
NefIgArd was a Phase III, multicentre, randomised, double-blind, placebo-controlled two-part trial that tested the efficacy and safety of 9 months of treatment with Nefecon (16 mg/d) versus placebo in adult patients with primary IgAN at risk of progressing to kidney failure.16 NefIgArd enrolled adult patients with IgAN with persistent proteinuria (UPCR: ≥0.8 g/g or proteinuria: ≥1 g/24 hour) despite supportive care optimised to the maximum allowed or maximum tolerated dose for at least 3 months, and an eGFR of ≥35 to ≤90 mL/min per 1.73 m2. Exclusion criteria included inadequately controlled blood pressure (i.e., systolic blood pressure/diastolic blood pressure: ≥140/90 mmHg), previous kidney transplant, and all secondary forms of IgAN, or any non-IgAN glomerulonephritis.16
Part A of the Phase III NefIgArd Trial Confirms the Findings of the Phase IIb NEFIGAN Study
An interim readout (Part A) was performed in November 2020, when the first 199 patients had received treatment for 9 months.16 This study had a prespecified primary endpoint of 24-hour UPCR, and a key secondary endpoint of eGFR. At 9 months, UPCR was 27% lower in the Nefecon group compared with placebo (p=0.0003). UPCR continued to improve in patients treated with Nefecon; at 12 months there was a 48% reduction in UPCR with Nefecon compared with placebo (p<0.0001). Prespecified analyses demonstrated that in the subgroup of patients with baseline UPCR ≥1.5 g/g, the eGFR benefit was greater in the patients treated with Nefecon compared with the overall population. These results confirmed the findings of the Phase IIb NEFIGAN study, and led to Nefecon being the first drug to be approved by the U.S. Food and Drug Administration (FDA)17 and the European Medicines Agency (EMA)18 for the treatment of patients with IgAN at risk of disease progression.
Part B of the Phase III NefIgArd Trial Shows Efficacy and Safety over 2 Years
The full study results (Part B; n=364) were presented at the ERA Congress.15 The aim was to confirm the long-term renal benefit of the observed reduction in proteinuria. At baseline, the median age was 43 years in the Nefecon group and 42 years in the placebo group, while approximately two-thirds of patients were male (64.3% in the Nefecon group and 67.6% in the placebo group). The median UPCR at baseline was 1.28 g/g in the Nefecon group and 1.25 g/g in the placebo group, and the median UACR was 0.99 g/g in the Nefecon group and 0.98 g/g in the placebo group. The median eGFR Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) was 56.14 mL/min/1.73 m2 in the Nefecon group and 55.11 mL/min/1.73 m2 in the placebo group at baseline. The frequency of systemic corticosteroids of immunosuppressants before randomisation was 8.2% in the Nefecon group and 10.4% in the placebo group. The median time since IgAN diagnosis was 2.4 years in the Nefecon group and 2.6 years in the placebo group.
The primary endpoint was the time-weighted average change in eGFR from baseline over the study period. The results demonstrated a 5.05 mL/min/1.73 m2 eGFR treatment benefit in favour of Nefecon versus placebo over 2 years (p<0.0001; Figure 1). This confirmed that the eGFR benefit of 9 months of active treatment with Nefecon was maintained during the observational follow-up. The placebo group demonstrated a decline in kidney function, despite being on optimised supportive care. Patients in the placebo group lost 5.85 mL/min/1.73 m2 of eGFR in 1 year, with a 12 mL/min/1.73 m2 decrease over 2 years. Treatment with Nefecon halved the degree of kidney function loss over the 2-year period.
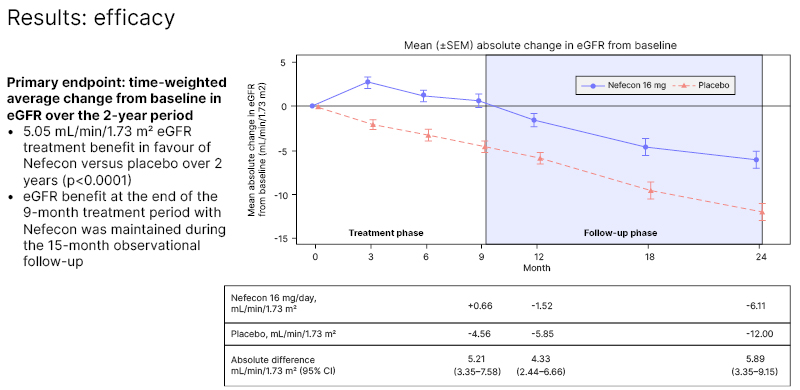
Figure 1: Mean (±standard error of the mean) absolute change in estimated glomerular filtration rate from baseline.15
CI: confidence interval; eGFR: estimated glomerular filtration rate; SEM: standard error of the mean.
The trial also showed that the eGFR benefit with Nefecon versus placebo was consistent regardless of baseline UPCR, meaning that treatment had a protective effect on kidney function in patients with proteinuria above or below 1.5 g/g.
At 2 years, the percentage reduction in UPCR in the Nefecon versus placebo arm was similar to the percentage reduction at the end of the 9-month treatment period (Figure 2). Patients treated with Nefecon experienced decreasing levels of proteinuria while on active treatment and for 3 months afterwards, suggesting a continued biologic effect. From 12–24 months, the level of proteinuria started to increase in those treated with Nefecon, but remained 30–40% lower than at the start of treatment. The open-label extension study will provide further information, when patients are restarted on a new treatment session.
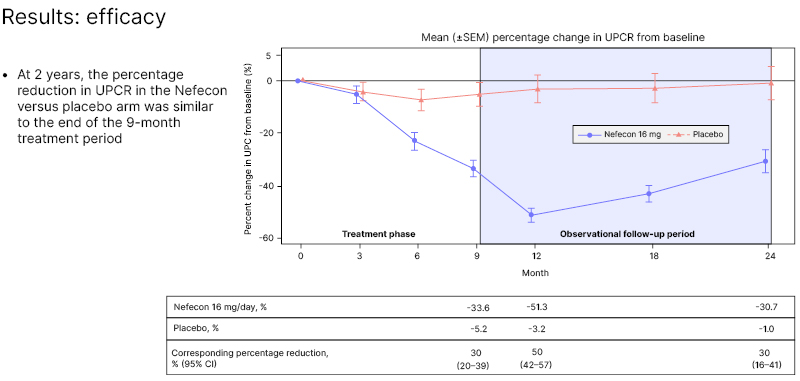
Figure 2: Mean (±standard error of the mean) percentage change in urine protein-creatinine ratio from baseline.15
CI: confidence interval; SEM: standard error of the mean; UPCR: urine protein-creatinine ratio.
Treatment-emergent AEs were as expected with low systemic exposure of a corticosteroid.15 Importantly, these events occurred while on active treatment, and were reversible when patients stopped taking the drug. In contrast, the efficacy data showed a continued benefit of treatment for a full 2-year period, including only 9-month exposure to the drug. Biomarker analyses from the NefIgArd Phase III clinical trial are ongoing. The results showed that treatment with Nefecon reduces circulating levels of Gd-IgA1 in patients with IgAN.19
What Do the Results Mean for Clinical Practice Guidelines?
The impact of IgAN can be illustrated using UK registry data in more than 4,000 paediatric and adult patients with IgAN.20 The data show that, despite being treated with the current standard of care for IgAN, three-quarters of adults and half of paediatric patients develop kidney failure or die within 20 years of disease onset.
In addition, at least 50% of patients diagnosed with IgAN below the age of 50 years are on dialysis before age 60 years. This highlights that while 20 years may seem like a long gap before poor outcomes are experienced, the reality is that kidney failure and death occur while patients are still in their 50s.
The registry data was also used to model the likelihood of developing kidney failure in a patient’s lifetime, dependent on prespecified rates of decline of eGFR. This showed that 100% of patients diagnosed at age 30–<40 years, with an annual eGFR decline of 3–5 mL/min/1.73 m2, would develop kidney failure in their lifetime. At an annual eGFR decline of 2 mL/min/1.73 m2, 80% would experience kidney failure. For context, the placebo group in most studies deteriorated at 5–6 mL/min/1.73 m2 per year. Real-world data showed that at an eGFR decline of 3 mL/min/1.73 m2, all patients below the age of 50 years developed kidney failure and needed dialysis in their lifetime.
Kaplan–Meier curves of the registry data showed that one in four patients with time-averaged proteinuria 0.5–1.0 g/24 hours developed kidney failure by 10 years (Figure 3). This indicates that it should be reconsidered whether patients achieving <1 g/day are sufficiently treated with supportive care.
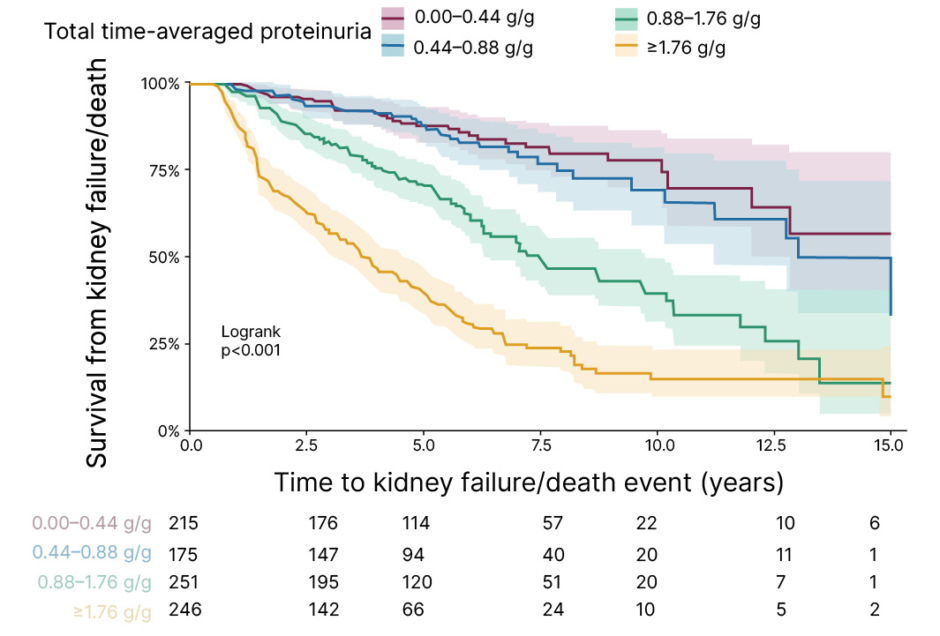
Figure 3: Kaplan–Meier survival curve of time to kidney failure/death using total follow-up time-averaged proteinuria.
Patients with IgA Nephropathy at Risk of Disease Progression Should Be Treated Early and Aggressively
In summary, a diagnosis of IgAN should be viewed in the context of the patient’s lifetime, not just the next 5 or 10 years, and treatment should be given to prevent kidney failure in the patient’s lifetime. A new mindset is needed of preserving nephrons as soon as diagnosis is made. This suggests that the current treatment paradigm21 needs to change towards one that is more focused and aggressive. This could include:
• A foundation of optimised kidney care with RAS inhibitors for all patients with proteinuric kidney disease. The use of sodium-glucose cotransporter-2 inhibitors, endothelin receptor antagonists, and mineralocorticoid receptor antagonists may also be considered. This treatment will not modify the disease or stop IgA deposition.
• An anti-inflammatory agent, such as a complement inhibitor, for patients with inflammation in the kidney biopsy, as uncontrolled inflammation drives the loss of nephrons.
• All patients will also need a therapy that is directed at B cells and switches off the production of pathogenic IgA. Currently, there are strong data that Nefecon can deliver that goal, as it turns off the production of pathogenic IgA within the mucosal immune system. Repeated treatment with Nefecon to maintain low levels of Gd-IgA1 immune complexes may be required. Studies are ongoing with other potential therapies, including anti-CD38, APRIL, and B cell activating factor/APRIL inhibitors, and results are awaited.
The new paradigm would see all three elements of treatment initiated simultaneously in a multi-targeted approach, based on the current understanding of the pathophysiology of IgAN.
The ultimate aspiration is a precision medicine approach to the treatment of IgAN. Biomarker studies are underway that may help clinicians to direct the right drug to the right patient at the right time in the natural history of the disease.
Question and Answer Session
The symposium concluded with a lively question and answer session. One delegate commented on how Fellström’s presentation indicated a 20-year history leading up to the regulatory approval of Nefecon, and congratulated the investigators for successfully completing that marathon. There was an observation from India that perhaps systemic absorption with existing budesonide formulations used to treat inflammatory bowel disease is higher than that presented by Fellström, who had noted that 90% of Nefecon is cleared in first-pass metabolism by the liver, which minimises systemic side effects. Barratt clarified that Nefecon is a very different formulation of budesonide, which currently exists to treat inflammatory bowel disease, and the two cannot be compared due to different pharmacokinetics and absorption.







