Abstract
Background: More than 50% of patients have renal involvement in systemic lupus erythematosus (SLE) and may present different patterns according to glomerular, tubulointerstitial, and vascular involvement. Lupus vasculitis (LV), the least frequent renal vascular lesion encountered in SLE, is a serious clinicopathological entity rarely described, and its therapeutic guidelines are not formally defined.
Case presentation: The authors present a case of a 44-year-old woman with SLE admitted to the hospital with a history of general tiredness, worsening of polyarthralgia, hypertension, oedema, and chest pain with new kidney failure, hematuria, and proteinuria. A kidney biopsy was performed, establishing the diagnosis of Class IV lupus nephritis associated with LV, and intravenous cyclophosphamide was started according to the CYCLOPS protocol. She developed orthopnoea associated to peripheral oedema, leading to a diagnosis of lupus myocarditis, and the treatment was adjusted to mycophenolate mofetil with rituximab, resulting in the resolution of symptoms.
Conclusion: LV is a rare condition, and there are no consensus or protocol guidelines
for the treatment of this pathology, whose severity requires an emerging diagnostic
and therapeutic approach.
Key Points
1. Lupus vasculitis (LV) is a rare but severe manifestation of systemic lupus erythematosus, significantly influencing prognosis and requiring urgent diagnostic and therapeutic interventions.
2. This case report presents a 44-year-old patient with Class IV lupus nephritis and associated LV, managed with cyclophosphamide, rituximab, and mycophenolate mofetil.
3. Recognising and managing LV is critical, as it poses unique diagnostic and therapeutic challenges, necessitating tailored, multidisciplinary approaches to improve patient outcomes.
INTRODUCTION
More than 50% of patients with systemic lupus erythematosus (SLE) develop lupus nephritis (LN) during the disease, which may manifest with various patterns involving the glomerular, tubulointerstitial, and vascular involvement. It is estimated that around 10–40% of patients with LN present vascular lesions on renal biopsy, which often serve as a marker of poor prognosis.1-3
Despite several morphological patterns that have been identified, LV is characterised by the rarity of the histological pattern found, limited descriptions in the literature, and its severity, which requires an urgent diagnostic and therapeutic approach. LV is marked by an inflammatory infiltrate of neutrophils and mononuclear leukocytes of the intima and media of the vessels, with fibrinoid necrosis and rupture of the elastic lamellae on optical microscopy. In immunofluorescence there is strong staining for antigens related to fibrin in the vascular wall, with weak and variable staining for immunoglobulins and complement.1,2,4 The treatment of LN depends on the histological class identified in the renal biopsy and is primarily focused on addressing glomerular pathology. However, there is no consensus regarding the therapeutic approach to LV.1,4
CASE REPORT
The authors present a 44-year-old woman with a personal history of SLE diagnosed in 2018 with joint, skin, haematological, and serous involvement usually medicated with prednisolone (5 mg daily) and hydroxychloroquine (400 mg daily), without kidney function changes (serum creatinine: 0.8–1 mg/dL) or in the urine test.
She presented to the hospital in January 2023 due to general fatigue, worsening of polyarthralgia, hypertension, generalised morning oedema, and chest pain with pleuritic characteristics that had been ongoing for a month. On physical examination, she was hypertensive, tachycardic, exhibited marked peripheral oedema, and had decreased breath sounds at the lung bases. Laboratory findings indicated anaemia (haemoglobin: 7.7 g/dL), worsening of kidney function (urea: 108 mg/dL; serum creatinine: 3.2 mg/dL), and urine analysis revealed haematuria and proteinuria, with urinary sediment showing 39 erythrocytes/field with dysmorphic erythrocytes and 24 leukocytes/field, proteinuria 4.9 g/24h, and albuminuria 3 g/24h. Immunological studies highlighted complement consumption (C3: 25.8 mg/dL; C4: 2.7 mg/dL; C1q: 2.6 mg/dL), positive antinuclear antibodies (1/1280), positive anti-dsDNA antibodies (576.25 IU/mL), and negative antineutrophil cytoplasmic antibodies, as shown in Table 1.
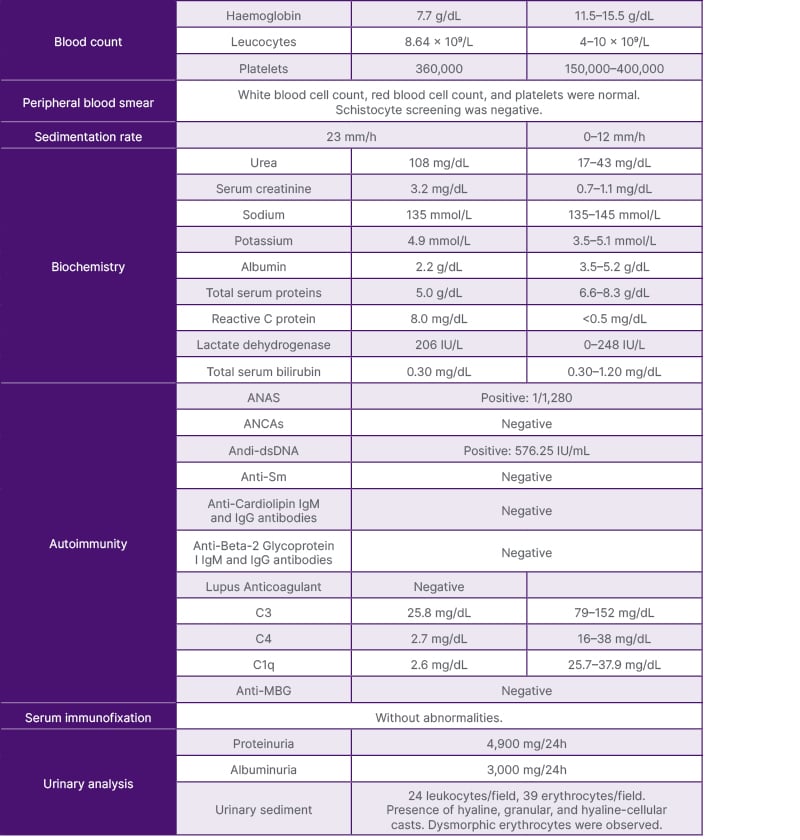
Table 1: The patient’s laboratory results at admission.
ANA: antinuclear; ANCA: antineutrophil cytoplasmic antibodies.
The kidney ultrasound revealed no significant changes, while the chest CT and echocardiogram showed bilateral pleural effusion and pericardial effusion with preserved systolic function, respectively.
LN was assumed to be probable, and intravenous methylprednisolone pulses (500 mg for 3 consecutive days) were administered, followed by prednisolone (1 mg/kg/day). After achieving clinical and analytical stabilisation, a kidney biopsy was performed (Figures 1 and 2), revealing the following:
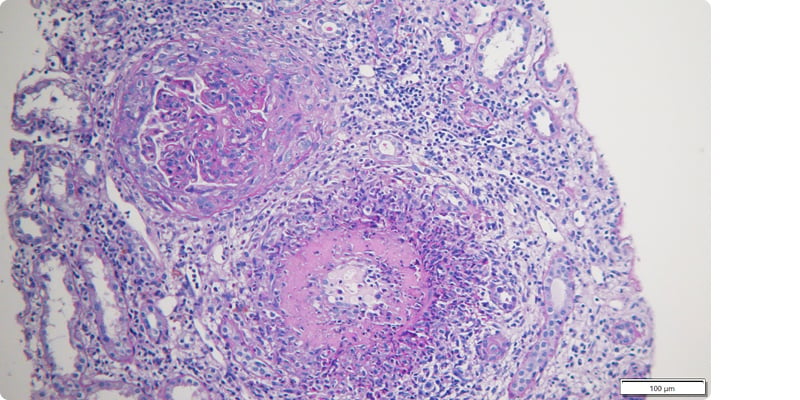
Figure 1: Glomerular crescent formation and capsule rupture in lupus nephritis.
Periodic acid-schiff staining, 100× magnification.
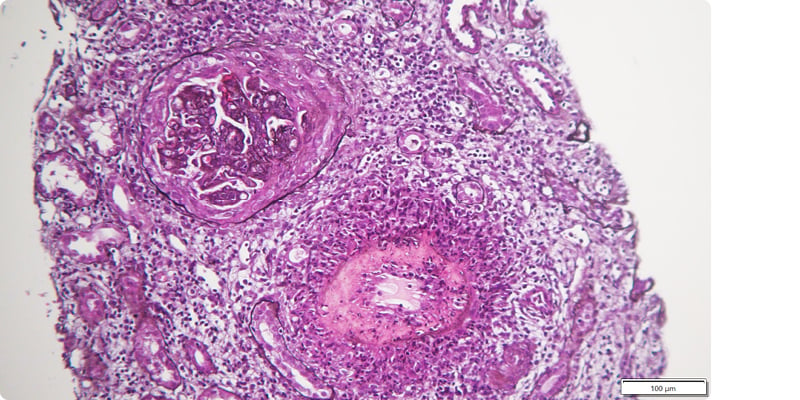
Figure 2: Fibrinoid necrosis and transmural inflammatory infiltrate in lupus vasculitis.
Silver staining, 50× magnification.
- Optic microscopy with nine glomeruli, of which two with global sclerosis, six with crescents (five cellular and one fibrocellular), with rupture of the capsule, carriorexis, endocapillary proliferation with polymorphonuclear cells, and subendothelial deposits (wire loops). Interstitium with intense global infiltrate and a band of interstitial haemorrhage. Two medium-sized arteries, one with fibrinoid necrosis of the wall and intense transmural inflammatory infiltrate with abundant polymorphonuclear cells surrounding the artery, and the other with mononuclear infiltrate.
- Immunofluorescence with mesangioparietal glomerular deposits of IgG++, IgM++, C3++, C1q+, Kappa++, and Lambda++. Circumferential fibrinogen deposition in the wall of a medium-sized artery. Test for negative amyloid substance.
A diagnosis of Class IV LN was established, with an activity index of 21/24 and chronicity index of 1/12 associated with LV. Intravenous cyclophosphamide (CYC) treatment, adjusted to the patient’s age and kidney function (12.5 mg/kg), was initiated following CYCLOPS protocol. However, there was a progression of kidney damage (urea: 157 mg/dL; creatinine: 4 mg/dL) despite improvement in proteinuria (7.2 g/24h), resolution of anaemia, decrease in anti-dsDNA (108 IU/mL), and increase in complement (C3: 50.7 mg/dL; C4: 12.8 mg/dL; C1q: 22.4). After completing the fourth cycle of CYC (cumulative dose of CYC: 2,750 mg), the patient began to experience new generalised fatigue, orthopnoea, and worsening of peripheral oedema. An echocardiogram was performed, which revealed new-onset impaired global systolic function with an estimated left ventricular ejection fraction of 43% along with elevated levels of B-type natriuretic peptide (>10,000 pg/mL). A CT angiography was performed, excluding acute coronary syndrome. Subsequently, cardiac magnetic resonance and PET scans were conducted, both highly suggestive of lupus myocarditis, leading to the decision to omit myocardial biopsy. After a multidisciplinary discussion, immunossupressive treatment was adjusted to mycophenolate mofetil (3 g/day) and rituximab (1 g, two administrations at 15-day intervals). Nine months after presentation, patient reassessement exhibited resolution of symptoms, improvement in kidney function (serum creatinine: 2.9 mg/dL, proteinuria: 2g/24h), and cardiac imaging and laboratory tests also showed significant improvement of cardiac dysfunction.
DISCUSSION
LN can present various histological patterns depending on disease activity and kidney involvement, which influence the clinical presentation and dictate the appropriate therapeutic approach. Thus, kidney biopsy plays a crucial role in characterising the disease and defining therapeutic and prognostic criteria.5
Given the predominant vascular involvement with marked disease activity, and the absence of consensus guidelines or randomised studies regarding the treatment of LV, considering the severity of the disease, it was decided to initiate immunosuppression with higher doses of CYC, in accordance with the protocol CYCLOPS, rather than following the treatment recommended for LN Class III/IV according to the EuroLupus protocol.5,6 It’s important to note that, in this case, drawing any conclusions regarding the effectiveness of the chosen treatment is challenging due to the necessity of changing therapy given the course of the disease.
It is worth emphasising that the measurement of antineutrophil cytoplasmic antibodies was negative. Although these antibodies are mainly associated with primary vasculitis, they can also be associated with vasculitis secondary to systemic connective tissue diseases. It is crucial to consider these overlapping conditions to avoid misdiagnosis.6
During treatment with CYC, the patient developed heart failure, prompting consideration of various aetiologies. Among the differential diagnoses, cyclophosphamide toxicity was deemed unlikely due to the cumulative dose being below the cardiotoxic range. Additionally, the temporal association between the worsening of left ventricular dysfunction and the renal disease flare suggested a different underlying cause. The imaging findings were not indicative of cyclophosphamide toxicity, as there was no restrictive pattern observed, and the left ventricular function appeared to normalise within 1 week under immunosuppressive therapy. Ischaemic heart disease was also considered; however, coronary angiography did not reveal any abnormalities consistent with thisa etiology. The findings documented on cardiac MRI fulfilled the modified Lake Louise criteria, further supported by evident myocardial inflammation observed on PET imaging, rendering myocardial biopsy unnecessary. In this context, treatment with mycophenolate mofetil was initiated in combination with rituximab, which according to guidelines should be considered in patients with persistent active disease or consecutive flares.7
Therefore, it is imperative to recognise the vascular component of lupus in clinical practice and to encourage further research into the therapeutical management of this serious and rare clinical entity.







