Abstract
Introduction: Intradialytic hypotension (IDH) still remains a common finding in maintenance haemodialysis despite improvements in dialysis delivery. Measures are needed to minimise some aftermath of IDH like dialysis termination, which can impact poorly on dialysis outcome.
Methods: This retrospective study assessed IDH in a low-income setting, and compared two cohorts of IDH with and without dopamine treatment.
Results: Of the 416 participants, 92 (22.1%) had at least an episode of symptomatic IDH. Of these, 20 (21.7%) were treated with dopamine. Of the 2,205 sessions, 468 (21.2%) had symptomatic IDH, of which 63 (13.4%) with severe IDH were treated with dopamine. The mean age of all participants and dopamine treatment participants were 50.8 ± 9.3 years and 64.6 ± 9.5 years, respectively (P=0.001). Blood pressure (BP) reductions following dialysis were more with females (P=0.04). Dialysis dose was adequate in 7.9% and 4.2% of sessions with and without dopamine (P<0.001). Improvements in glomerular filtration rate were greater in dopamine-treated sessions (P=0.03 and P=0.04, respectively). Fewer anti-hypertensives (aOR: 14.64; 95% confidence interval [CI]: 7.88–20.41), low predialysis systolic (aOR:5.59; 95% CI: 3.88–9.41), and diastolic blood pressure (aOR: 5.78; 95% CI: 4.06-9.81) were independently associated with dopamine-treated sessions.
Conclusion: IDH was found in 21.2% of dialysis sessions. 13.4% with severe IDH had dopamine treatment. Participants with dopamine-treated sessions had fewer dialysis terminations and hospitalisations, and dopamine treatment improved the prescribed dialysis and gave higher dialysis doses. Considering the economic effects of dialysis termination in low-income nations, intradialytic dopamine could be very beneficial.
Key Points
1. The prevalence of intradialytic hypotension (IDH) in Nigeria is reported to be 31.3% and 19.4% using The National Kidney Foundation’s Kidney Disease Outcome Quality Initiative guidelines.2. IDH is more prevalent in the presence of comorbidities, particularly cardiovascular disease.
3. Individuals with dopamine-treated sessions are more likely to have fewer dialysis complications, and a higher blood flow rate, ultrafiltration volume, and dialysis dose.
INTRODUCTION
Intradialysis hypotension (IDH) commonly complicates haemodialysis treatment, and could be associated with dysfunction of the central nervous, cardiovascular, and gastrointestinal systems; worsening of renal function; loss of vascular access; inadequate dialysis, poor quality of life; and death.1 The National Kidney Foundation’s (NKF) Kidney Disease Outcome Quality Initiative (KDOQI) guidelines in 2005 defined IDH as an intradialytic fall in systolic blood pressure (BP) ≥20 mmHg or of mean arterial pressure ≥10 mmHg, leading to emergence of symptoms.2
The prevalence of IDH is estimated to range from 8%–40% based on a wide range of diagnostic criteria involving BP values only, or with symptoms, or further still with nursing intervention.3-5 In the authors’ local environment, the prevalence of IDH is reported to be 31.3% and 19.4% using the NKF-KDOQI criteria, and 8.6% using the European Best Practices Guidelines (EBPG).6-8 The absence of a consensus definition has limited the scope of research on IDH and its outcome. However, Flythe et al.10 reported that the nadir BP was more predictive of future mortality of the eight IDH definitions they reviewed.
With ultrafiltration, inadequate compensation by the heart, blood vessels, and splanchnic bed that mediate plasma refilling and/or augmentation of the venous return leads to IDH.11 The compensatory activation of the sympathetic nervous system and the renin angiotensin aldosterone systems mediates vasoconstriction, leading to increased resistance in the peripheral, renal, and splanchnic circulations, and resulting in increased venous return, preload, and the cardiac output.12, 13
Despite advances in dialysis delivery, IDH is reported to be frequent in about 8% of the dialysis population.14 IDH is more prevalent in the presence of comorbidities, particularly cardiovascular disease, hence KDOQI recommended that patients on maintenance haemodialysis should have a cardiovascular assessment with an echocardiogram every 3 years.15 Heart failure is reported to increase the risk of frequent hospitalisation and poor outcome in treatment with inotropes like dopamine, just as dopamine is reported to be ineffective, or even harmful, when used to treat cardiac failure or acute kidney injury.16-18 Pharmacological-based strategies used in managing IDH in the past included enhancing left ventricular relaxation in patients with left ventricular hypertrophy (LVH) using verapamil, reducing the heart rate using atenolol, decreasing the predialysis systolic BP using amlodipine, and stimulating α-1 adrenergic receptor agonist using droxidopa.19-22 Other measures taken in the past included using carvedilol in the BLOCADE pilot study,23 and increasing the numbers of BP-lowering drugs in patients with poorly controlled BP, in a Japanese study.24 Despite other benefits that were seen in these studies, they were all reported to be non-effective in improving IDH.
Adenosine A1 receptor antagonist, FK352, was associated with improved rates of IDH, similar to anti-diuretic hormone (ADH) that allowed higher ultrafiltration rates.25,26 The increased serum osmolality associated with thirst and increased interdialytic weight gain limited its continued use.27. Midodrine, an α-1 adrenergic receptor agonist prodrug, given predialysis, improved IDH and increased nadir systolic BP, but had no effect on the dialysis dose.28 Its usefulness prompted the American Society of Nephrology (ASN) to contest midodrine’s withdrawal by the U.S. Food and Drug Administration (FDA).29,30 Dobutamine had also be reportedly used in managing frequent IDH, with some successes.31
Dopamine, a naturally occurring adrenergic agent, is used in medical, surgical, and most commonly in intensive care units to manage hypotension and shock.32,33 In Nigeria, as in many low-income nations (LIN), about 90% of the dialysis population is not on any health insurance scheme.34-36 A commonly feared side effect of dopamine is tachycardia, seen mostly in its medium and high doses, and around which many of its other adverse effects like arrhythmias and cardiac toxicity are hinged.37 Dopamine use in treating IDH is scarcely reported. The authors hypothesise that low-dose dopamine regimen is effective and safe in managing IDH. This study compared sessions with IDH with and without dopamine treatment.
MATERIALS AND METHODS
This was a 3-year retrospective cohort study in which the dialysis sessions of patients between 16–78 years old, with CKD diagnosed according to the KDOQI 2012 criteria,33 who received maintenance haemodialysis between August 2018–July 2021 at the dialysis suite of Babcock University Teaching Hospital, Ilishan-Remo, Nigeria, were studied. The sessions were grouped into three cohorts as no IDH, IDH without dopamine (IDHWD), and dopamine-treated sessions (DTS).
Participants’ case notes and dialysis chats were retrieved, and variables obtained were age, gender, cause and type of kidney disease, percent oxygen saturation (SPO2) pulse rate (PR) BP predialysis and, every quarter of an hour throughout dialysis. Also retrieved were the number of hospitalisation, comorbidities, total dose of dopamine per dialysis, and duration of dopamine use per dialysis. The results of pre- and postdialysis renal biochemistry, electrocardiogram (ECG), and echocardiogram were also retrieved.
Excluded were sessions with predialysis dopamine infusion, intradialysis hypertension, or sessions with other inotropes.
Inclusion criteria for dopamine treatment was ≥three consecutive episodes of severe IDH (intradialysis drop in SBP ≥20 mmHg to <100 mmHg with symptoms, and in which nursing interventions were unsuccessful leading to dialysis termination, after ruling out and/or correcting modifiable factors such as fever, drug effect, or food intake).34-36 Dopamine 2-5 ug/kg/min in 200 ml of 0.9% saline was commenced whenever the SBP fell by ≥20 mmHg or SBP <90 mmHg, with symptoms such as nausea, yawning, cramps, dizzy spells, syncope, body pains, and/or chest discomfort that did not respond to routine treatment measures. Intradialysis anticoagulation was with unfractionated heparin (5,000 IU). The dialysate flow rate (DFR) was 500 ml/min for all sections, and the dialysate sodium, potassium calcium, and bicarbonate were 140 mmol/L, 2.0 mmol/L, 2.0 mmol/L, and 34 mmol/L, respectively. Whenever sodium profiling was carried out, the mean dialysate sodium concentration was documented.
The study was approved by the Babcock University Human Research Ethics committee (BUHREC/723/19, NHREC/24/01/2018).
DEFINITIONS
Tachycardia:
- Mild (PR: 101–119/min)
- Moderate (PR: 120–139/min)
- Severe (PR: 140–149/min)
- Life-threatening (PR ≥150/min)37
Hypoxaemia:
- SPO2 <95%38
Dopamine:
- Low dose: <5 ug/kg/min
- Medium dose: 5–9 ug/kg/min
- High dose: ≥10 ug/kg/min39
Targeted weight loss: Predialysis weight plus volume of administered fluid minus UFV40
IDH: ≥20 mmHg intradialysis fall in SBP1
Severe IDH: ≥3 consecutive episodes of intradialytic drop in SBP≥20 mmHg to <100 mmHg with symptoms, in which nursing interventions were unsuccessful leading to dialysis termination (after ruling out and/or correcting modifiable factors such as fever, drug effect, or food intake), requiring the need for inotropic support36
Anaemia: hematocrit <33%41 Hypoalbuminaemia: <35 mg/dl42
Dialysis dose: Normal (Kt/V ≥1.2), low (Kt/V 0.9–1.1), and very low (Kt/V <0.9)43
Hypertension-associated CKD: Longstanding hypertension that led to kidney disease common in elderly and late middle-aged patients
Chronic glomerulonephritis: Kidney disease complicated by hypertension, common in the young and in early middle age, with or without antecedent history of pharyngitis or skin sepsis
In this study, hospitalisation is defined as hospital admission lasting up to 24 hours. All 557 participants had an ECG, but only 43 (7.72%) had an echocardiogram, on account of cost.
STATISTICAL ANALYSIS
Data was analysed using SPSS version 22.0 (IBM, California, USA). Continuous variables with means and standard deviations were compared using T-test. Categorical variables as proportions and percentages were compared using Chi-square test or Fisher’s exact test when variables were less than five. The P value <0.05 was considered statistically significant. Variables with P <0.025 were entered into a multiple regression model to determine predictors of dopamine use in IDH, using backward elimination to adjust for confounders.
RESULTS
Two thousand two hundred and five sessions by 416 participants were studied. Ninety-two (22.1%) participants had ≥1 episode of symptomatic IDH. Of the participants with symptomatic IDH, 20 (21.7%) were treated at least once with dopamine. Of the 2205 sessions, 1737 (78.8%) had no IDH, 468 (21.2%) had symptomatic IDH, and of this, 63 (13.4%) with severe IDH had dopamine treatment (Table 1). The mean age of all participants, participants with no IDH, participants with IDHWD, and with DTS were 50.8 ± 9.3 years, 49.6 ± 7.5 years, 53.8 ± 8.7 years, and 64.6 ± 9.5 years, respectively (P=0.001). The mean age of the 11 (55.0%) males and 9 (45.0%) females with DTS were 63.8 ± 7.7 years, and 65.5 ± 8.3 years (P=0.04).
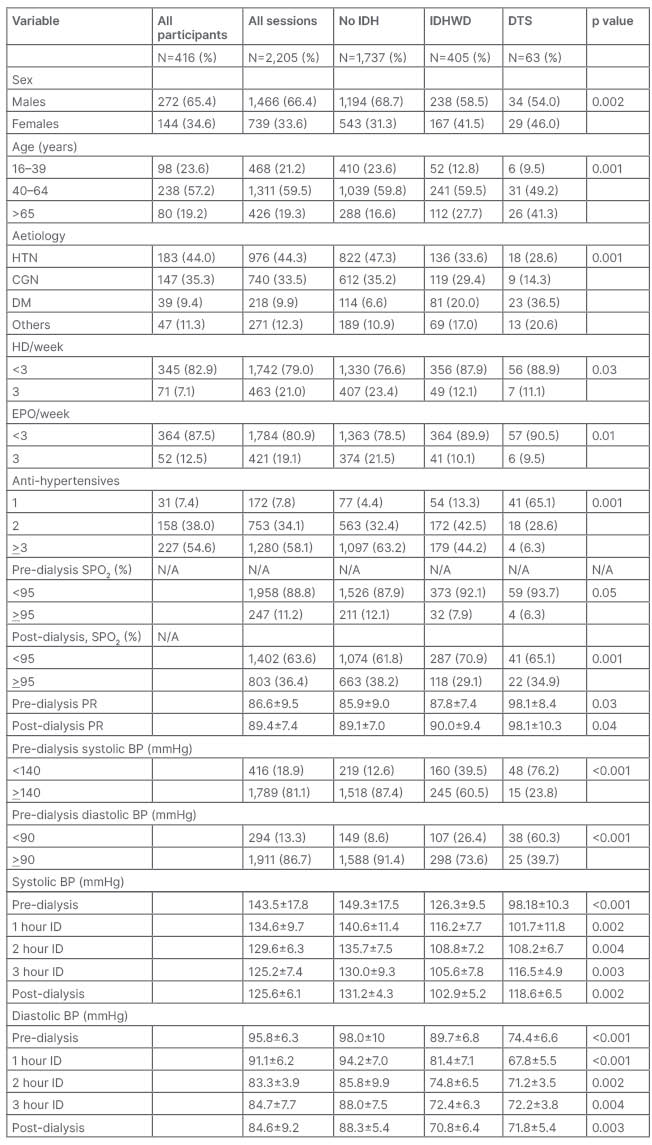
Table 1: Sociodemographic, historical, and clinical characteristics of study population.
BP: blood pressure; CGN: chronic glomerulonephritis, DM: diabetes; DTS: dopamine-treated sessions; EPO: erythropoietin; HD: haemodialysis; HTN: hypertension; ID: intradialysis; IDH: intradialysis hypertension; IDHWD: intradialytic hypotension without dopamine; PR: pulse rate; SPO2: percent oxygen saturation.
Predialysis, DTS had more hypoxaemia compared to sessions with IDHWD (P=0.05). Postdialysis, sessions with IDHWD had more hypoxaemia than DTS (P=0.001). The mean predialysis systolic BP of males and females with DTS were 119.3 ± 7.5 mmHg versus 118.3 ± 5.9 mmHg (P=0.13); postdialysis, these were 120.0 ± 22.9 mmHg and 117.5 ± 22.8 mmHg (P=0.04). The mean predialysis diastolic BP of males and females in DTS were 75.3 ± 4.6 mmHg and 73.3 ± 4.0 mmHg (P=0.05); postdialysis, these were 75.3 ± 5.9 mmHg and 69.5 ± 22.8, P=0.001. The dialysis dose was adequate in 335 (15.2%), 313 (18.0%), 17 (4.2%), and five (7.9%) of all sessions, sessions without IDH, sessions with IDHWD, and DTS, respectively (P<0.001). Three hundred and twenty nine (79.1%) participants had LVH using the Sokolow–Lyon criteria on the ECG. Of the 43 participants that had echocardiogram, eight (18.6%) had ejection fraction <50%, 33 (76.7%) had concentric LVH, four (9.3%) had diastolic dysfunction, one (2.3%) had systolic dysfunction, and 27 (81.8%) had combined systolic and diastolic dysfunction. The 18 (4.3%) participants with echocardiogram confirmed heart failure had 8.1% of the sessions with IDH but without dopamine, while 14.3% had IDH with dopamine treatment. The 39 (9.4%) participants with diabetes had 20.0% of the sessions with IDH but without dopamine, while 36.5% had IDH with dopamine treatment.
Following dialysis, the rise in mean serum sodium and fall in mean urea were more with DTS than sessions with IDHWD (P=0.07 and P=0.05, respectively). The rise in mean SBC and fall in mean potassium were more in sessions with IDHWD than DTS (P=0.09 and P=0.1, respectively). There was a greater fall in mean anion gap in DTS compared to sessions with IDHWD (P=0.04). The fall in mean serum creatinine and the rise in mean GFR was more with DTS than IDHWD (P=0.04 and P=0.03, respectively). There was a greater increase in the hematocrit in DTS than sessions with IDHWD (P=0.05). The mean yearly hospitalisation per participant for all, those without IDH, participants with IDH without dopamine, and those with dopamine were 2.73 ± 1.23, 2.67.11 ± 1.21, 2.94 ± 1.24, and 2.88 ± 1.22, respectively.
In the multiple regression analysis, fewer antihypertensives (aOR: 14.64; 95% CI: 7.88–20.41; P<0.001) low predialysis systolic BP (aOR: 5.59; 95% CI: 3.88–9.41; P-0.001), and low predialysis diastolic BP (aOR: 5.78; 95% CI: 4.06–9.81; P-0.001) were independently associated with DTS (Table 2 and Table 3).
DISCUSSION
The incidence of symptomatic IDH was 21.2%; of this, 13.5% of patients with severe IDH were managed with dopamine. The mean blood flow rate, ultrafiltration volume, dialysis duration, and the dialysis dose were higher in DTS, while the frequency of dialysis termination was less with the DTS compared to sessions with IDHWD. The risk of intradialytic death was marginally higher in the DTS. Females, the elderly, and diabetics were more likely to develop IDH, and to require dopamine treatment. Participants with predialysis hypoxaemia were more likely to develop IDH and require dopamine. Under-dialysis, lesser erythropoietin use, fewer antihypertensives, and lower predialysis BP were associated with IDH and dopamine use.
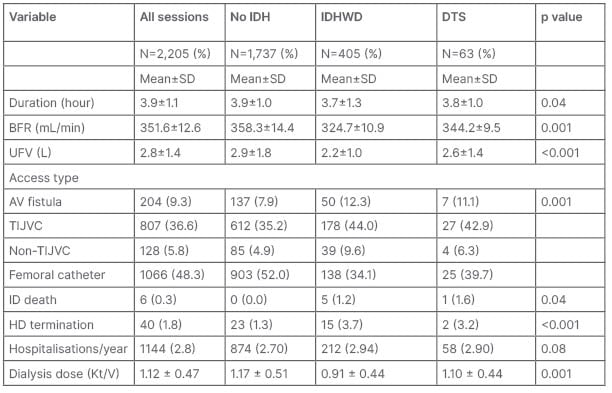
Table 2: Dialysis prescription, intradialytic events, and outcomes in patients.
AV: arterovenous; BFR: blood flow rate; DTS: dopamine-treated sessions; HD: haemodialysis; ID: intradialytic; IDH: intradialytic hypotension; IDHWD: intradialytic hypotension without dopamine; TIJVC: tunneled internal jugular vein catheter; UFV: ultrafiltration volume.
The frequency of symptomatic IDH falls within the very wide range reported in previous studies, but higher than the 10.1% reported by Kuipers et al.44 for the EBPG, and lower than the 31.3% found in a study in Nigeria.6 The lower rate in the EBPG compared to this study is expected, considering the association of several limiting factors in LINs that would normally entail higher rates of dialysis complications like IDH. One would have expected a higher rate compared to findings by Okpa and his group,6 since intradialysis hypertension was excluded in this study, but the subjective nature of symptom reportage and perhaps the acceptance of these by the dialysis team could differ widely between centres.10
The 13.4% prevalence of severe IDH in this study falls within the 8% and the 17.2% classified as frequent IDH by Kuipers et al.,44 and Sands et al.45 The 20 participants with DTS met the 20% of dialysis sessions cut-off criteria of the EBPG, for diagnosis of frequent IDH. The non-responsiveness of their dialysis sessions to routine IDH treatment regimen, except with inotropic support, is in agreement with Allapan et al.,46 who managed severe IDH with midodrine.
The mean dialysis duration of the DTS was higher than the sessions with IDHW, and this reflected the lower risk of dialysis termination in the DTS. This agrees with findings by Anandh et al.,31 who reported that dobutamine was effective in reducing the episodes of severe IDH (SBP <90 mmHg), admission rates, and left ventricular ejection fraction. The higher BFR in the DTS compared with the IDHWD in this study agrees with Cruz et al.,47 who reported that with midodrine (an inotrope), higher BFR could be achieved, as the increased risk IDH from a rising BFR is prevented with an inotropic support that increases the heart rate, peripheral resistance, and the cardiac output through increased sympathetic activities. The higher UFV in the DTS than the IDHWD reflects the ability of inotropes to prevent the BP reduction that would have been enough to meet the BP diagnostic criteria of IDH.32, 33
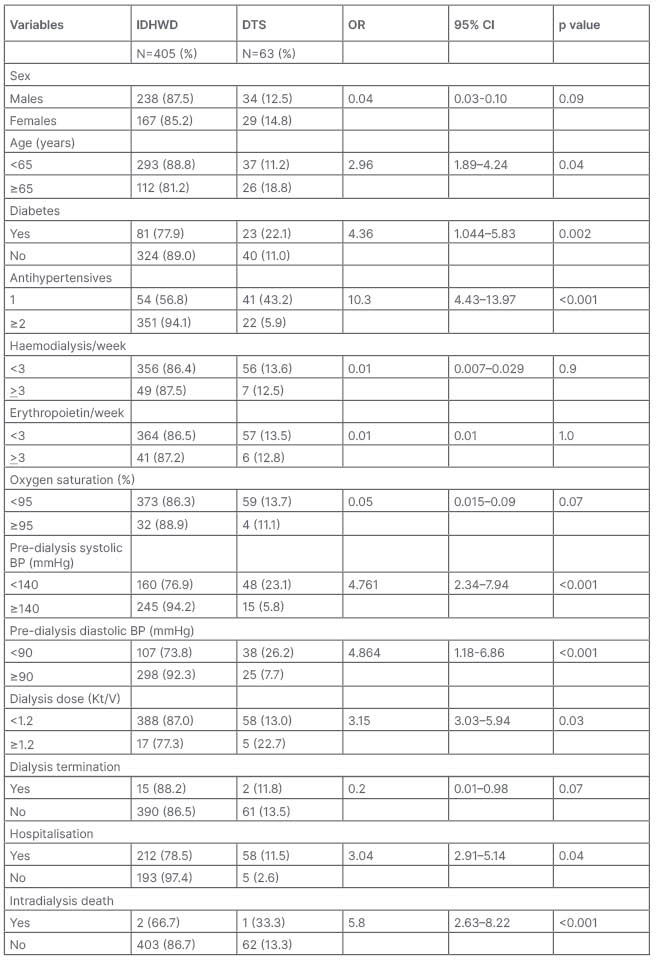
Table 3: Relationship between dialysis dose and correlates of intradialysis hypotension.
BP: blood pressure; CI: confidence interval; DTS: dopamine-treated sessions; IDH: intradialysis hypotension; IDHWD: intradialytic hypotension without dopamine. OR: odds ratio.
The higher dialysis dose in the DTS is not in agreement with previous findings.23 Renal dose dopamine mediates vasodilatation and increased intradialysis renal blood flow, augments residual renal function contributes to solute and water clearance and dialysis dose.34,35 This is less significant with dobutamine which mediates more of chronotropic activities leading to higher cardiac output, thereby reducing the risk of IDH, but with lesser effect on renal blood flow and residual renal function.31
The combination of longer dialysis duration, and higher BFR and UFV in the DTS compared with IDHWD, explains the higher dialysis dose. The smaller BP difference in the DTS following dialysis confers a lesser risk of ischaemic organ dysfunction. It could reduce the incidence of dialyser blood clotting and vascular thrombosis.48-50
Though all deaths in sessions with IDH (with and without dopamine) were associated with intradialytic hypotension, it is worth noting that the only death in DTS was that of a 62-year-old female with disseminated ovarian cancer.51 Choi et al.,52 in a review of several findings from studies involving dopamine use associated with kidney function, suggested a nephroprotective effect of dopamine. Low-dose dopamine acting on the D-1 and D-2 like receptors induces sodium and water excretion, increases renal perfusion and stabilises BP, all of which are treatment targets in kidney disease.
The inverse relationship between the frequency of dialysis and IDH could be attributable to the shorter interdialytic periods, lesser interdialytic weight gain, lower osmotic gradients, lesser UFV, and therefore lower risk of IDH.53 The inverse relationship between the number of BP lowering drugs and the risk of IDH (and dopamine use), tend to suggest either a cardiac decompensation or autonomic dysfunction particularly baroreceptors mediated, as strictly renal diseases, though rare, commonly present with poor BP control, particularly in end stage disease, and more so in the Black population.54 However, this relationship depicts more of an ‘effect’ rather than a ‘causal’ one, as the increased risk and/or the occurrence of IDH necessitated the reductions in the number of anti-hypertensives.
Fewer erythropoietin use, commonly associated with severe anaemia, is complicated by higher plasma volume that would necessitate higher UFV.55 Similarly, higher viscosity from frequent erythropoietin use would be more likely to induce sluggish flow, dialyser blood clotting, and elevated blood pressure, and at times dialysis termination.56 Agarwal et al.57 in their findings noted an positive relationship between the hematocrit and the risk of IDH.
Dialysis termination is not uncommon, and could be a very distressing occurrence in LINs when one considers the difficulty some of the patients pass through to secure funds for a dialysis session.6-8 The practice of fresh payment for every new dialyser, that is common in almost all dialysis centres in this clime, makes all attempts at preventing dialysis termination a worthwhile task.6 The greater risk of IDH and dopamine treatment in diabetes and heart failure in this study agrees with previous findings, and could be associated with neuropathy-induced neurovascular abnormalities and cardiac remodelling, leading to diastolic and systolic dysfunction associated with lower cardiac output and ejection fraction.58,59 However, the relative absence of tachycardia would have limited any unwanted effects associated with these diseases.33
The better treatment outcome of participants that were treated with low-dose dopamine in this study was mostly secondary to increased BP that allowed for higher BFR, UFV, and longer dialysis time. This all led to higher dialysis dose, and lower dialysis termination and hospitalisation rates, on a background of mild or no tachycardia, as were reported in previous studies.29,30
Several limitations were encountered in this study. The retrospective design, the absence of newer devices for monitoring/treating IDH like hematocrit monitoring, bioimpedance, and biofeedback ultrafiltration were unavailable. Participants’ dry weight and residual kidney function were not assessed during dialysis sessions. The dialysis suite had no dialysate cooling machine. Cardiac enzymes (troponins and creatinine kinase-MB) were not assayed. The blood PH, the best measure to assessed metabolic acidosis, was not assessed. The inclusion of IDH prone participants added to the strength of this study.
CONCLUSION
Intradialytic hypotension is still common. The authors found a frequency of 21.2%, and of this 13.4% had severe IDH requiring inotropic support. Diabetes, heart failure, female gender, advancing age, low predialysis blood pressure, fewer dialysis, and erythropoietin treatment were associated with IDH and dopamine treatment. Participants with DTS were more likely to have fewer dialysis terminations and hospitalisations, and higher BFR, ultrafiltration volume, and dialysis dose. Considering the economic effect of dialysis termination on the dialysis population in LINs, the use of low-dose intradialytic dopamine infusion (which has the added advantage of increasing the dialysis dose) could be of benefit.








