Abstract
Membranous nephropathy (MN) is the major cause of nephrotic syndrome in adults, accounting for 20% of cases with an annual incidence of 1 per 100,000 population. In the past 10 years, the role of podocytes has been identified. Environmental triggers in genetically predisposed patients can activate podocytes to exhibit antigenic epitopes, including PLA2R, THBS1, and NELL1, which become targets of specific autoantibodies with subsequent complement activation. The discovery of these mechanisms has opened a new horizon in the treatment of MN, and novel drugs are available with more specific mechanisms of action. Rituximab, a monoclonal antibody directed against CD20 expressed on B lymphocytes, has been used in several trials and appears to induce remission of nephrotic syndrome in 60% of patients (GEMRITUX trial). The recently published results of the MENTOR trial documented the superior efficacy of rituximab in patients observed for up to 24 months. In MN, the concept of targeting disease control has introduced novel therapies with specific blocking mechanisms, such as belimumab; nonspecific blocking mechanisms, such as those against adrenocorticotropic hormone; and new therapeutic options, such as ofatumumab, bortezomib, and eculizumab, which have recognised the pathological processes involved in the glomerular diseases.
INTRODUCTION
Membranous nephropathy (MN) is the most frequent cause of nephrotic syndrome in adults and older patients. It accounts for 20% of nephrotic syndromes in adults and its annual incidence is 1 per 100,000 population. Overall in Europe, 10,000 new cases are diagnosed per year.1 In the last 10 years, the pathogenetic mechanisms have been defined which has opened up new ways of treatment.
RESEARCH METHODOLOGY
This paper reviews the latest insights into the pathogenesis and treatment of MN. The authors analysed available papers on MN pathogenesis and MN therapy by performing a literature search using PubMed with the search terms “MN pathogenesis” and “MN therapy.” Firstly, the papers published in the last 3 years were examined. Paper selection was made according to the relevance of the journal, the authors, the dimension of the study, and the novelty of the findings, with 20 recently published papers selected. The authors then looked to older publications, and studies previously published were also included. Currently ongoing studies were searched for in “https://clinicaltrials.gov/” and randomised controlled trials (RCT) that were active and enrolling patients were included. Those that had not started or were closed were not included. Overall, the story of MN is a very long one and it has not yet finished. This literature review covers from the first studies in 1995, up to the time of writing.
AETIOLOGY AND PATHOGENESIS
In recent years, MN has been found to be essentially a disease of the podocyte which, as a response to environmental triggers and on a genetic basis, exhibits antigen epitopes which bind antibodies that are then able to bind complement. The first antigen to be recognised was NEP by Debiec et al.2 Later, a different podocyte protein, the M-type PLA2R, was identified as the antigen responsible for 70–80% of MN.3 Because PLA2R is a normal molecule of the podocyte structure, MN may be regarded as an autoimmune disease, at least in those patients in whom anti-PLA2R antibodies may be found.4 The discovery of M-type PLA2R as a major antigen in idiopathic MN (iMN) was a breakthrough in the understanding of the pathogenesis of this disease, establishing iMN as an autoimmune disease. Subsequent studies confirmed that circulating antibodies against PLA2R were detected in approximately 70% of incident iMN patients. Recently, it has been shown that the presence of PLA2R antibodies supported a diagnosis of iMN, changes in antibody levels were related to clinical disease activity, disappearance of antibodies preceded and predicted a subsequent decrease of proteinuria, and high titres of antibodies were associated with a low likelihood of spontaneous remission.5 Recently, another podocyte antigen, THSD7A, has been found to be responsible for around 10% of MN.6
In a recent study of PLA2R-negative patients with MN, the technique of laser dissection of glomeruli followed by mass spectrometry allowed the identification of the presence of the protein NELL-1 that was also localised by immunohistochemistry.7 The authors concluded that a subset of MN is associated with co-localisation of NELL-1 and IgG along the glomerular basement membrane. In these patients, anti-NELL antibodies were also found in the serum. Additionally, the antigens of aldose reductase and superoxide dismutase have been suggested in some cases of MN.8 As a consequence of these findings, for a better understanding of the disease and of possible new therapies, studies are now looking for new podocyte markers that are able to activate the complement cascade and for cells that are able to produce the antibodies involved.9,10
The Toronto Glomerulonephritis Registry determined the Toronto Risk Score, dividing patients into those at low risk for progression, intermediate risk, and high risk, according to their proteinuria levels in the first 6 months.11 van den Brand et al.12 found markers predictive of evolution towards renal failure in a1 and b2 microglobulin excretions.13 The best marker of the disease evolution and determinant for treatment is the titre of anti-PLA2R.14-16
TREATMENT
Supportive Treatment
In the first period of the disease, supportive treatment without the use of immunosuppressants is recommended by the Kidney Disease Improving Global Outcomes (KDIGO) guidelines for all patients with MN and nephrotic syndrome.17 Recommended treatment consists of restricting dietary sodium intake to <2 g/day and controlling blood pressure, hyperlipidaemia, and oedema. For all patients, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers should be the first-line therapy because of their antiproteinuric effect.18 If the nephrotic syndrome persists, or patients have a recurrence of nephrotic syndrome in the first 6 months of treatment, immunosuppressive treatment should be considered.
Immunosuppressive Treatment
The combination of corticosteroids with cyclophosphamide or chlorambucil, given over 6 months, is best-known as ‘Ponticelli’s regimen.’ Several studies have documented a remission rate of 70–80% with this treatment.19-22 Cyclophosphamide and chlorambucil are equally effective in inducing remission, but one study21 has documented a better tolerability profile for cyclophosphamide. A prophylactic treatment with trimethoprim sulfamethoxazole is recommended to avoid pneumocystis pneumonia.23
Anti-Calcineurin Drugs
Prospective, randomised studies have documented the efficacy of cyclosporine A (CsA) and tacrolimus (TAC) in the treatment of MN.24-26 In addition to their immunosuppressive effect, CsA and TAC have an antiproteinuric effect because of their action on podocyte structure via interaction with synaptopodin.27 The main drawbacks of CsA and TAC are their nephrotoxicity and the high rates of recurrence when the drugs are reduced.28
Mycophenolate Mofetil
Several observational studies have suggested that mycophenolate mofetil (MMF) is effective for the treatment of MN.29 However, the only published controlled study did not document such efficacy.30 For this reason, although the combination of MMF with a high dose of corticosteroids appears effective, the KDIGO guidelines do not recommend MMF as the first-line of treatment for patients with MN.17
Rituximab
Rituximab (RTX) is a monoclonal antibody directed against CD20 on the surface of B lymphocytes. In the case of MN, RTX is used to block the production of antibodies directed against the antigens that characterise the MN. Previous observational studies documented the efficacy of RTX in MN, with complete or partial remission of the nephrotic syndrome in 60% of patients.31-34 No treatment-related serious adverse events were reported in either study. The RTX doses used in the different studies have principally been 375 mg/m2/week for 4 weeks, or one or two 1g doses. More recently, a controlled, prospective, randomised trial commenced (GEMRITUX trial),35 comparing two doses of 375 mg/m2 with supportive treatment versus supportive treatment alone in 75 patients with MN. The study results at 17 months documented a remission rate for patients treated with RTX of 65% versus 34% in supportive treatment alone (p<0.03).
INSIGHTS FROM THE MENTOR STUDY
The recent publication of the first 24 months of data from the MENTOR study has allowed for better clarification of the role and relevance of RTX in the treatment of iMN.36,37 The MENTOR study is a landmark investigator-initiated, open-label, randomised, noninferiority trial conducted in North America. In the study, 130 patients with primary MN, almost all positive for PLA2R, were randomised to receive RTX or CsA. The primary endpoint was complete remission or partial remission of proteinuria at 24 months post-randomisation.
Patients assigned to receive RTX were given a dose of 1 g on Day 1 and on Day 15. If complete remission was attained at 6 months, no additional RTX was given, but if proteinuria decreased by >25% from baseline at 6 months, a second course of RTX was given. When proteinuria did not decrease by >25% at 6 months, it was considered a treatment failure. Patients assigned to CsA were given 3.5 mg/kg/day in two equally divided doses. If complete remission was attained at 6 months, CsA was stopped. If proteinuria decreased by >25% from baseline at 6 months, CsA was continued for an additional 6 months. A treatment failure was considered when proteinuria decreased <25% at 6 months. All patients were followed for 24 months.
The cumulative treatment failure for each treatment is shown in Table 1. The difference in favour of RTX is both high and significant, particularly at 24 months. Table 2 shows the response at 24 months according to baseline anti-PLA2R levels, and again documents a significant response in favour of RTX. Overall, the MENTOR study documented that RTX was not inferior to CsA in inducing complete or partial remission of proteinuria and was superior in maintaining proteinuria remission up to 24 months.
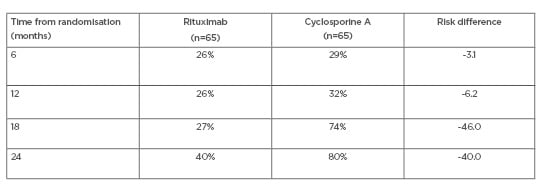
Table 1: MENTOR trial: cumulative treatment failure.
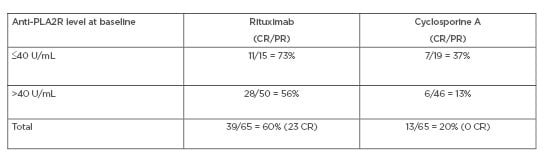
Table 2: MENTOR trial: baseline anti-PLA2R levels and response at 24 months.
CR: complete remission; PR: partial remission.
WHAT SHOULD BE THE INITIAL THERAPY FOR PRIMARY MEMBRANOUS NEPHROPATHY?
The first-line, initial therapy should be either RTX or cyclophosphamide (the latter according to Ponticelli’s regimen). A cyclophosphamide regimen may be preferred for patients with very high levels of anti-PLA2R, even if the clinical response does not seem to be predicted by PLA2R at baseline. There is currently an Italian RCT underway, the RICYCLO trial,38 comparing RTX to cyclophosphamide. CsA is now considered the second-line therapy for MN, with MMF, Acthar® gel, and plasma exchange as third-line therapies.
What Can Be Done to Augment the Response to RTX?
Higher doses of RTX can be used when anti-PLA2R levels are very high. Combination treatment of RTX with low-dose cyclophosphamide for 3–6 months may be considered, but a RCT is needed. Ofatumumab, obinutuzumab, or other drugs mentioned below may be used to augment the response to RTX but, again, an RCT is needed. Adjunctive plasma exchange or immunoadsorption39 may also be considered, or combination therapy with sequential calcineurin inhibitors, as is undergoing testing in the STARMEN trial.40
How do Anti-PLA2R Antibody Levels Influence the Treatment of Primary MN?
This issue was not tested in the MENTOR study because no placebo control or stratification for anti-PLA2R levels was undertaken. However, from the available data, high PLA2R levels were associated with higher resistance to treatment, while low levels were associated with a high spontaneous remission rate. In addition, declining serum anti-PLA2R levels predicted clinical remission, while rising levels predicted relapse. However, CsA was much less effective in lowering anti-PLA2R antibody levels.
Unanswered Questions
The efficacy and safety of RTX in comparison to cyclophosphamide-based regimens is unanswered by the MENTOR study, but the RI-CYCLO trial aims to determine this. It is unclear from the MENTOR study what the influence of anti-PLA2R antibody status is on the efficacy of RTX because no stratification for anti-PLA2R levels was made. The study does not clarify if there is any clinical value in monitoring CD19/20 B cells in circulation. Finally, the study does not answer the question as to whether RTX therapy will prevent end-stage renal disease. Box 1 summarises the insights from the MENTOR Study, as well as the unanswered questions.

Box 1: Insights and unanswered questions from the MENTOR study.
CD: cluster of differentiation; CsA: cyclosporine A; CyC: cyclophosphamide; ESRD: end-stage renal disease; MN: membranous nephropathy; RTX: rituximab.
Ongoing Trials with Rituximab
There are several ongoing trials comparing RTX with cyclophosphamide or anti-calcineurin drugs, using different RTX doses. There are four ongoing RCT with RTX. The peptide GAM immunoadsorption therapy in autoimmune membranous nephropathy (PRISM)41 study is based on the understanding that iMN is an autoimmune disease characterised by the presence of IgG autoantibodies to M-type PLA2R. Immunoadsorption is a method of removing specific circulating immunoglobulins and has been shown to remove >80% of circulating IgG with a single session of 2.5 plasma volumes, with albumin and antithrombin III unaffected; with multiple sessions this can rise to >98%. The sequential treatment with TAC–RTX versus steroids plus cyclophosphamide in patients in the iMN(STARMEN) trial40 will compare a TAC–RTX treatment with Ponticelli’s regimen-treated groups. Rates of remission, relapse, and preservation of renal function will be evaluated at a 2-year follow-up.42 Trial NCT0097797743 compares RTX to CsA. The CsA group will withdraw CsA after 6 months and introduce RTX. The RI-CYCLO trial38 is recruiting MN patients in Italy to compare the efficacy of RTX with Ponticelli’s regimen.
ADRENOCORTICOTROPIC HORMONE
Adrenocorticotropic hormone (ACTH) stimulates the production of endogenous glucocorticoids and activates the melanocortin receptors, which perform several functions including immunomodulation, anti-inflammation, and modulation of exocrine functions.44 In rodents, these receptors have been found in podocytes and glomerular endothelial, mesangial, and tubular epithelial cells. In animal models affected by iMN, the inhibition of melanocortin receptors reduces proteinuria and improves podocyte morphology.45 After a first pilot study,46 two studies47,48 demonstrated the beneficial effects of natural ACTH in resistant glomerular diseases. Hladunewich et al.,49 in a prospective open-label study, confirmed these beneficial results in 20 patients affected by iMN. To date, two ongoing studies are registered on clinicaltrials.gov.50,51
NEW EXPERIENCES
Ofatumumab
Ofatumumab is a new monoclonal antibody acting on CD20. It differs from RTX because it has different target epitopes. Ofatumumab, in addition to acting on the same epitope as is recognised by RTX, also acts on a second epitope localised on the small loop of CD20 and on a portion of the large, extracellular loop. Ofatumumab has been assessed as a RTX rescue therapy. Ruggenenti et al.52 recently described two cases of clinical remission of iMN in patients who developed primary and secondary resistance to RTX. Resistance to RTX in these cases could be as a result of a change in the CD20 antigen conformation, which prevents B-cell–RTX binding and the consequent B-cell depletion.
Belimumab
As a monoclonal antibody, belimumab specifically targets the soluble form of B-lymphocyte stimulator that has a critical role in the differentiation and homeostasis of B lymphocytes. The effects of belimumab on proteinuria and anti-PLA2R antibody production have been evaluated in 14 patients with anti-PLA2R-positive MN. The treatment significantly reduced the antibody titre and proteinuria within 12 weeks.53 Changes in proteinuria and in anti-PLA2R antibody titre after belimumab treatment seemed to parallel the changes observed after RTX, with a delay in onset. This may reflect the immediate B-cell lysis achieved by RTX, whereas the slower effect of belimumab might reflect the progressive ‘exhaustion’ of antibody-producing B cells secondary to B-lymphocyte stimulator binding and inhibition.
Targeting Memory Plasma Cells
The advanced stages of MN could be mediated primarily by autoreactive plasma cells, which are resistant to anti-CD20 monoclonal antibodies but sensitive to anti-CD38 antibodies or proteasome inhibitors.52 Memory plasma cells survive RTX because they do not express the CD20 antigen; plasma cells express CD38.54,55 These autoreactive plasma cells could be a target for anti-CD38 monoclonal antibodies, such as daratumumab and isatuximab. To date, these agents have been developed to kill malignant plasma cells.55 Other molecules, such as the proteasome-inhibitor bortezomib, may effectively deplete plasma cells. Bortezomib acts by causing an intracellular accumulation of abnormal proteins with consequent plasma cell apoptosis. To date, bortezomib has been used in antineutrophil cytoplasmic antibody nephritis56 and in resistant systemic lupus erythematosus.57 Preliminary data suggest it may be useful in the treatment of iMN that is resistant to other therapies.58,59 The main drawback of bortezomib is its toxicity, which necessitates treatment interruption in most patients.
Targeting Complement
Complement inhibition by the anti-C5 monoclonal antibody eculizumab could be another avenue for treating iMN. In this approach, complement inhibition could prevent glomerular damage that occurs prior to the removal of antibodies.60 To date, attempts to treat membranous nephropathy by eculizumab have failed and studies remain unpublished.
CONCLUSION
The discovery of anti-PLA2R antibodies and other antibodies involved in the pathogenesis of MN has revolutionised the treatment approach to this disease; for the first time, iMN may be considered an autoimmune disease in which podocytes play the initial and most important role. The possibility of monitoring anti-PLA2R antibodies represents an important tool for nephrologists to monitor the disease and to check the therapeutic effects. Other autoantibodies have been recognised as causative in iMN, including autoantibodies against THSD7A and NELL-1. Few iMN remain to be explained, but it is likely only a short time until other antigens and autoantibodies will be discovered.
After the publication of the first data of the MENTOR trial, RTX represents the most important drug in the treatment of iMN. Several relevant questions remain to be answered. What is the most appropriate dosage? What is the role of other immunosuppressants? What is the role of PLA2R or other autoantibodies at baseline? How should relapses be treated? Several ongoing RCT aim to answer these questions. Additionally, the pharmaceutical pipeline is filled with other new drugs which are all the subject of RCT. Ofatumumab for MN resistant to RTX, drugs targeting the memory plasma cells, and drugs affecting the complement pathway seem to be the most important therapies for future study and potential treatment of MN.








