Abstract
The importance of extracellular volume control and avoidance of volume overload has been well documented in relation to the management of patients with chronic haemodialysis. Chronic volume overload results in poorly controlled hypertension, increased cardiovascular events, and increased all-cause mortality. Traditional methods of dry weight assessment have relied on clinical assessment to guide volume status. The challenge of achieving the balance between dry weights and preventing intradialytic complications is a formidable one. In order to achieve this, reproducible and sensitive methods are desirable to aid objective quantification of volume status. One such method is by the use of blood volume monitoring, which is achieved by real-time calculation of changes in relative blood volume via a cuvette placed in the arterial blood-line, which can be used to guide ultrafiltration targets during the haemodialysis session. This review article examines the use of blood volume monitoring as a tool to guide ultrafiltration during dialysis and to examine the current evidence to supports its use in assessing dry weight and in preventing intradialytic hypotension events.
INTRODUCTION
The importance of extracellular volume control and avoidance of volume overload have been well documented in relation to management of patients with chronic haemodialysis. Chronic volume overload results in poorly controlled hypertension, increased cardiovascular events, and all-cause mortality.1–3 Traditional methods of dry weight assessment have relied on clinical assessment to guide volume status. Unfortunately, relying on clinical signs of volume overload and assessment of dry weight correlates poorly with a true euvolemic state. Various studies have shown that up to 25% of patients in haemodialysis cohorts are chronically volume overloaded.4,5 Indeed, according to Agarwal et al.,6 markers of intravascular volume expansion, such as inferior vena cava diameter, blood volume monitoring (BVM), inflammatory markers, and plasma volume markers, may not be directly reflected by the clinical finding of oedema.7
The achievement of dry weight is associated with improvement in blood pressure control8-10 and reduction in the requirement for antihypertensive medication.5 Blood pressure control without the use of pharmacotherapy is a strong predictor of survival in the population on dialysis and hence dry weight achievement, by extension, is a positive prognostic factor.11 Conversely, aggressive ultrafiltration and targeting an inappropriately low dry weight can lead to intradialytic hypotension (IDH), nausea, central nervous system dysfunction, cramping, and risks compromising vascular access and worsening residual renal function.6,12
The challenge of achieving the balance between dry weight and preventing intradialytic complications is a formidable one. In order to achieve this, reproducible and sensitive methods are desirable and would aid quantification of volume status. One such method is the use of BVM, which is achieved by real-time calculation of changes to relative blood volume via a cuvette placed in the arterial bloodline. These calculations can then be used to guide ultrafiltration targets during haemodialysis sessions.4,13,15 This review article examines the use of BVM as a tool to guide ultrafiltration during dialysis and examines the current evidence to support its use in assessing dry weight and in preventing IDH events.
BLOOD VOLUME MONITORING IN PRACTICE
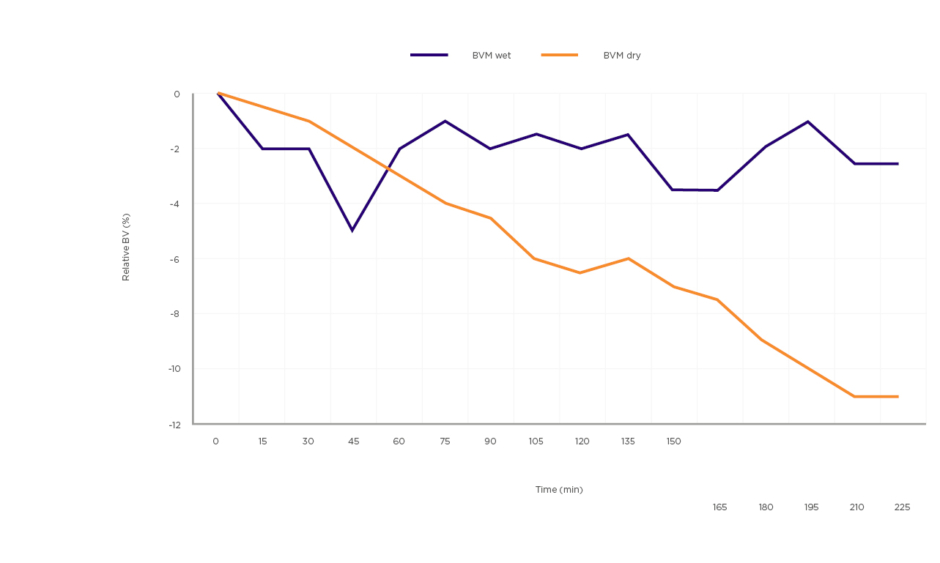
The use of BVM dates back to the early 1990s when CRIT-LINE® technology (Fresenius Medical Care, Bad Homburg, Germany) was first used as a non-invasive method of measuring haematocrit changes during haemodialysis, in real time, by connection of an additional monitor to the dialysis arterial line set-up.16 This technology has continued to evolve, with some haemodialysis platforms now including software that uses continuous BVM biofeedback to automatically optimise ultrafiltration during the treatment (HemoControl®on the Artis Pysio® system, Baxter, Deerfield, Illinois, USA). While the mechanism of each BVM system may differ slightly, the underlying fundamentals are similar. As ultrafiltration removes fluid from the intravascular space, it changes the haematocrit, concentration of protein, and overall density of the blood.17,18 Changes in the density of the blood can be determined by the velocity at which sound travels from the ultrasonic transmitter to the receiver; from this the relative blood volume (RBV) can be calculated within 2.9% accuracy.18-20 A flat BVM curve during a dialysis session suggests that the plasma refill rate is occurring at an equivalent or higher rate than ultrafiltration (UF). Hence, a flat curve signals that there is scope to further increase the UF target and adjust the dry weight of the patient in the right clinical setting. A ‘flat-curve’ has been defined as a <5% reduction in RBV during the course of treatment. For patients with a >5% drop in RBV, a plasma refill test can be conducted at the end of the dialysis session. This is performed by turning off UF and rechecking the RBV after ten minutes; a vascular refill resulting in a ≥1.5% increase in RBV is consistent with excessive refill from extravascular compartments, thus indicating volume overload.12 Patients with a >5% drop in RBV and a plasma refill of <1.5% are considered to have adequate UF and accurate dry weight goals.12,21-24 (Figure 1). An RBV critical level is also determined to guide the rate of UF and in theory prevent IDH events. RBV critical levels are calculated by documenting the RBV level at which a patient develops symptomatic hypotension.25 While BVM is useful in most patients on dialysis, one of the main limitations is its unreliability in patients with low UF rates (<2.5 mL/kg/hour).21
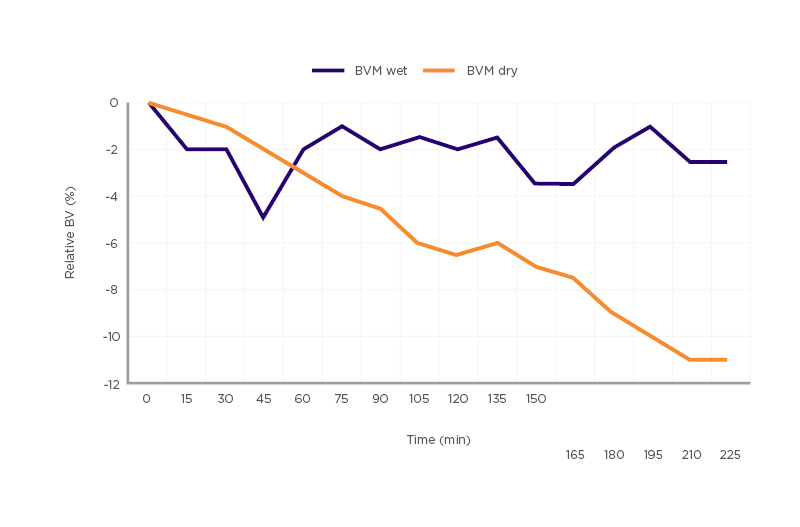
Figure 1: Comparison of blood volume monitoring wet versus blood volume monitoring dry.
A relative blood volume reduction of >5% and <1.5% relative blood volume plasma refill at the end of ultrafiltration treatment are classified as blood volume monitoring dry.
BVM: blood volume monitoring.
BLOOD VOLUME MONITORING AND DRY WEIGHT
The concept of dry weight dates nearly as far back as the invention of intermittent haemodialysis.7 The definitions of dry weight have changed over time but can be defined as the lowest post-dialysis weight tolerated without significant signs of hypovolaemia.6,16,17 The DRIP trial26 found that extracellular volume expansion may be present even in the absence of clinical signs. This supports the clinical practice of dry weight challenging as a first-line strategy to improve blood pressure control as extracellular expansion is often accompanied by hypertension. Studies have quoted the prevalence of volume overload in patients on dialysis to be as high as 25%, demonstrating the clinical burden it poses on dialysis management.4,5,27
When the importance of volume control is discussed it is important not only to correlate it with adequate dialysis goals but also to examine the long-term consequences of a chronically fluid-overloaded state. Patients with end-stage kidney disease are unable to maintain fluid and salt haemostasis and hence volume overload plays a key role in increased cardiovascular events in the population on haemodialysis.28-30 The intermittent nature of haemodialysis results in a constant flux between dry weight and intradialytic weight gain, which is associated with cyclical cardiac stress and ultimately cardiac remodelling.31 The persistent hypertension resultant from chronic volume overload lends itself to the development of left ventricular hypertrophy.32 Left ventricular hypertrophy causes both systolic and diastolic dysfunction, which predisposes patients to the risk of fatal arrhythmias.28,33 It is unsurprising that cardiovascular disease accounts for over half of all-cause mortality in the population on dialysis given the above and accelerated vascular calcification.34 Given the clinical significance of this issue, clinical research into developing strategies to aid judgement of appropriate dry weight is ongoing. One such strategy is investigating the effectiveness of BVM as a predictor of dry weight and appropriate goal-directed ultrafiltration.5,12,14,21-24
The data surrounding BVM as an effective tool in the management of ultrafiltration in patients on dialysis has yielded mixed results. The CLIMB trial14 hypothesised the use of Crit-Line technology to monitor intradialytic haematocrit would decrease patient morbidity in comparison to conventional methods based on symptoms, blood pressure, weight, and physical exam. However, results from the trial found that there was a greater number of hospitalisations and mortality in the Crit-Line interventional arm than the conventional arm of the trial. The authors advised that the results of the trial should be interpreted with caution as there may have been a failure to randomise clinical variants among the two study groups equally. However, the trial casts doubt whether quantitative monitoring via a BVM is superior to clinical judgement.
Hecking et al.23 described the use of BVM with regulation of ultrafiltration and dialysate conductivity (UCR) and/or regulation of ultrafiltration and temperature versus a conventional control group to decrease dry weight in fluid-overloaded patients on haemodialysis. The study attempted a rapid dry weight reduction in a volume-overloaded dialysis population. While the trial showed that there were fewer intradialytic complications in the ultrafiltration and temperature group (20±19%) versus UCR (47±27%) and the conventional group (41±30%), the overall complication rate remained high. Despite dealing with a population deemed volume overloaded, the rates of intradialytic hypotension mirrored that of the DRIP trial.26 The authors noted technical mistakes in 36% of UCR dialysis sessions and therefore the trial results are to be interpreted with caution. A 17±22 mmHg reduction in systolic blood pressure was noted following dry weight reduction; however, there was no significant difference between each of the groups. This suggests that dry weight reduction results in improved blood pressure control regardless of the modality used. Similar findings in blood pressure were also demonstrated regarding dry weight reduction in the DRIP trial.26
BVM is unable to directly define dry weight since intradialytic changes in blood volume only account for the plasma compartment.12,35 However, the extracellular compartment can mirror intradialytic changes reflected by the rate of vascular refilling.22,36 Rodriguez et al.22 hypothesised this in a study using Crit Line III monitors to assess dry weight. The trial theorised that intradialytic changes and post-dialytic refilling are both indirectly related to the composition of the extracellular compartment. Using Crit Line III monitors, all 28 patients in the trial had their dry weight adjusted from baseline assessment, 19 patients had their dry weight decreased, and nine patients had their dry weight increased. The changes to dry weight were based on post-dialytic vascular compartment refill and patient symptoms. The authors concluded that BVM, in conjunction with clinical assessment, was effective in achieving true dry weight. Similarly, Hussein et al.12 found a high prevalence of volume overload in their study population. Forty-three percent of the 169 patients assessed were noted to be BVM wet, defined as failure to drop blood volume below -5% or an increase in blood volume by 1.5% during vascular refill. As such, dry weights were adjusted to new dry weight targets based on BVM findings. Maduell et al.21 concluded from their study of 55 patients that BVM was effective in determining high levels of volume overload but was less useful in detecting low-to-moderate levels of fluid overload (Table 1).
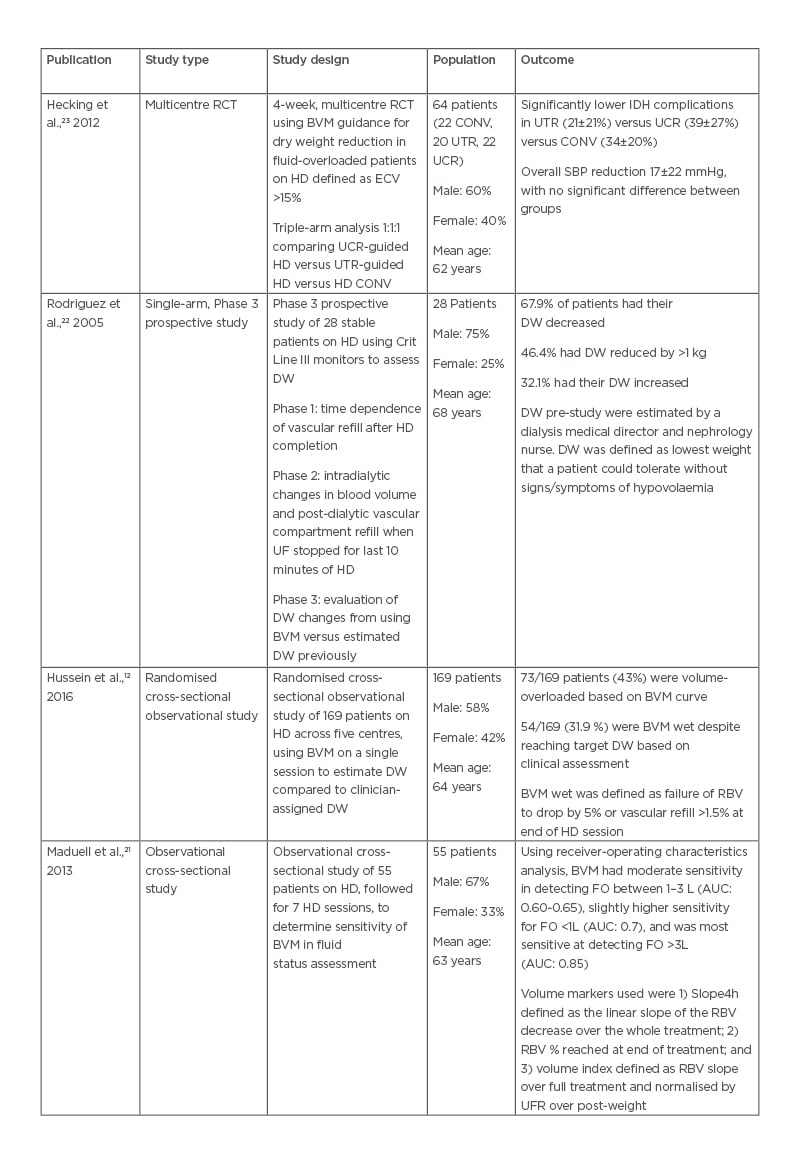
Table 1: Comparison of trial outcomes in utilisation of blood volume monitoring-guided ultrafiltration goals.
AUC: area under the curve; BVM: blood volume monitoring; CONV: conventional haemodialysis; DW: dry weight; ECV: extracellular volume; FO: fluid overload; HD: haemodialysis; IDH: intradialytic hypotension; RBV: relative blood volume; RCT: randomised controlled trial; SBP: systolic blood pressure; UCR: dialysate conductivity-regulated; UF: ultrafiltration; UFR: ultrafiltration rate; UTR: ultrafiltration- and temperature-regulated.
Given the range of results found within the literature, it is clear that the theory behind BVM doesn’t always correlate with findings in the patient population on dialysis. Achievement of dry weight can be hampered by intradialytic hypotensive episodes, which may not be solely related to intravascular volume status. Blood pressure changes during dialysis are multifactorial and include reduction in vascular tone and autonomic dysfunction, which are particularly important in the patient population who are on dialysis and diabetic.37
BVM AND INTRADIALYTIC BLOOD PRESSURE
Intradialytic blood pressure issues, predominantly intradialytic hypotensive events, are common among the population on dialysis with up to 30% of dialysis treatments complicated by intradialytic hypotensive events.38 Intradialytic episodes are not only a source of morbidity for patients but also have a significant impact on the efficacy of dialysis sessions, ultrafiltration goals, and cardiac dysfunction, and may compromise vascular access.39-41 The definition of intradialytic hypotension (IDH) is defined as ≥20 mmHg drop in systolic blood pressure accompanied by symptoms of hypoperfusion. Intradialytic hypotensive events have been associated with increased mortality, cerebral atrophy, myocardial stunning, ischaemic heart disease, and loss of residual renal function.6,12,42-47 The pathophysiology of IDH is multifactorial and includes a combination of changes in blood volume, reduced cardiac function, and failure of compensatory vasoconstrictive responses. Certain patient factors are associated with higher risk of IDH including diabetes, patients who are elderly and on dialysis, patients requiring longer haemodialysis sessions, and patients prone to autonomic dysfunction. Given the significance of intradialytic hypotensive events, a modality that could lead to the prediction or prevention of an event would be of great clinical benefit.49,50
Booth et al.13 conducted a study to assess the correlation between BVM and associated hypotensive invents in 72 patients on dialysis. While the results found that BVM correlated with changes in haematocrit, serum albumin, and extracellular fluid volume, the trends in BVM did not mirror intradialytic blood pressure. The data from this trial showed that there was no relationship between relative changes in BVM and intradialytic blood pressure. Similar results were also found by Leung et al.4 who conducted a 22-week, multicentre, randomised cross-over trial in 35 patients receiving regular intermittent haemodialysis who had >30% of sessions complicated by symptomatic IDH.4 Following a 4-week run-in period to allow standardised dry weight assessment, dialysis prescription review, and rationalisation of antihypertensive medications, patients were randomised into a control group (best clinical practice) or the intervention group (best clinical practice plus BVM). The BVM group adjusted for ultrafiltration rate but not dialysate sodium. The primary outcome of the trial was symptomatic IDH defined as ≥20 mmHg drop in systolic blood pressure from baseline accompanied by symptoms of IDH.
At the end of the trial period there was no difference in the incidence of IDH between the two groups.
Bégin et al.25 carried out a small study with more positive results for the use of BVM in the prevention of hypotension during haemodialysis. Seven patients on chronic haemodialysis with frequent IDH (>30% of dialysis sessions complicated by IDH) participated in a cross-over trial alternating between six consecutive sessions with blood volume regulation versus six standard dialysis sessions, for a total of 36 sessions. A dialysis session was considered event-free if symptomatic blood volume contraction did not occur, no sudden hypotensive event occurred, therapeutic intervention was not required, and departure from the dialysis unit proceeded as scheduled. The results showed a 74% increase in event-free sessions with use of BVM (50.8% versus 29.2% of sessions). While the results of this study had a positive result with the use of BVM to prevent hypotensive events, limited conclusions can be drawn given the small population involved.
De’ziel et al.51 studied hypertension control in a population on dialysis with ultrafiltration goals guided by BVM. The primary end-point was variation in baseline systolic, diastolic, and mean blood pressure from baseline to the end of the study. A secondary end-point was variation in baseline to the end of the study in the number of nursing interventions for IDH. This was a randomised controlled trial of 57 patients on chronic dialysis over a 6-month period. Patients were randomised to receive standard haemodialysis versus Hemocontrol® (HC) haemodialysis. Of the 44 patients who completed the trial (22 in each group), home blood pressure readings were available for 36 (19 in the standard haemodialysis group and 17 in the HC group). The trial showed a significant overall decrease in systolic blood pressure in both groups but no significant difference between the two groups (mean systolic blood pressure in the standard group decreased from 150.6 to 138.0 mmHg, and in the HC group systolic blood pressure reduced from 162.5 to 147.6 mmHg). However, on analysis of the secondary end-point, there was a significant reduction in the number of interventions required in the HC group versus the standard haemodialysis group. In addition, a quality-of-life questionnaire showed an improvement in the burden of kidney disease in the HC group while there was a deterioration in quality of life in the standard group. Overall, the literature presents mixed results for the use of BVM as a preventive measure for IDH. Further larger studies are needed to further assess its utility for this indication (Table 2).
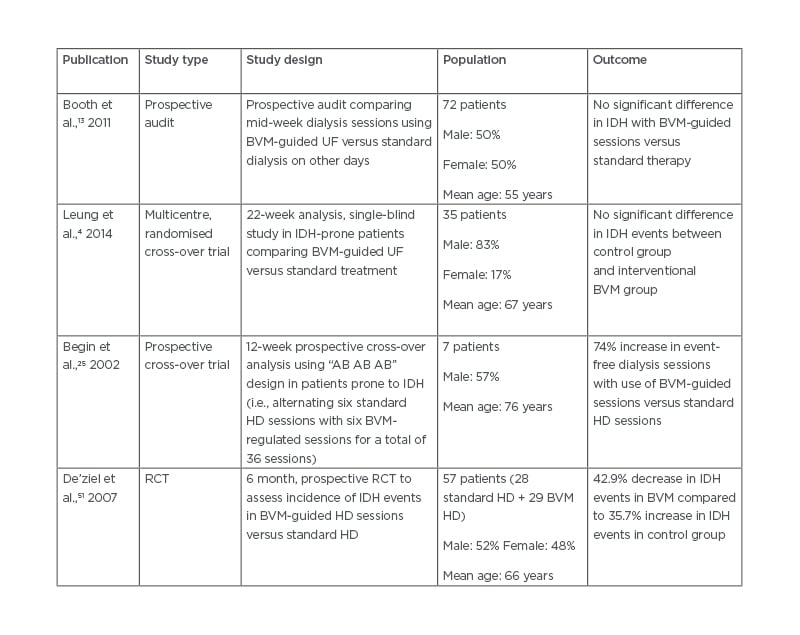
Table 2: Comparison of trial outcomes in blood volume monitoring utilisation in intradialytic hypotension prevention.
BVM: blood volume monitoring; IDH: intradialytic hypotension; HD: haemodialysis; RCT: randomised controlled trial; UF: ultrafiltration.
CONCLUSION
The use of BVM in both guiding ultrafiltration and preventing intradialytic hypotensive episodes has varied results in the literature. Given the significant interplay of physiological processes involved in volume control and haemodynamic changes in the population on haemodialysis, BVM may play a useful role in improving the efficacy and safety of care in addition to clinical assessment of patients, which is known to have its own limitations. However, further larger and more definitive trials, coupled with ongoing developments in technology, are needed to provide advances in this area.