Abstract
The incidence of diabetes in patient populations requiring dialysis is constantly increasing. Metabolic disturbances in this group need focussed attention, particularly as carbohydrate balance is affected by specific disease-related factors. Beta-cell dysfunction, insulin resistance, and advanced glycation end-product accumulation are increasingly detected in the period preceding dialysis. Glycaemic control is also linked to the health of bone metabolism and control of renal failure-related anaemia. Novel opportunities in the assessment of glucose homeostasis, including continuous glucose monitoring systems, skin autofluorescence, and investigation of the metabolome, have resulted in significant developments in diagnostics and therapy. Regarding antidiabetic control, the major therapeutic goal for patients on haemodialysis (HD) is the alleviation of glycaemic fluctuation during the post-dialytic phase. The periodicity in antidiabetic regimes on HD and non-HD days is the preferable tool. For patients on peritoneal dialysis, the adverse impact of glucose originated from the standard solutions should be counterbalanced. This review focusses on the relationship between diabetes and HD or peritoneal dialysis and provides clinical suggestions to support the planning of individualised therapy. Nowadays, the number of patients with advanced renal failure is increasing. In current medical training, nephrological and diabetic education is separated within the internal curriculum. Thus, an average nephrologist is not trained in diabetic issues that would enable them to control the carbohydrate metabolism of a patient with renal insufficiency at different stages of glomerular filtration rate narrowing, and additionally is not permitted to change the choice of therapy. Conversely, a general diabetologist is not aware of the effects of kidney failure and dialysis on glycaemic control and is not familiar with the technological details of renal replacement therapies: special alterations related to nephrological factors are therefore not taken into account when treating diabetic patients with kidney disease. The article deals with the theoretical and practical issues of this clinical border area, helping the clinician to choose individual treatment for a particular patient. Guidelines for choice of oral and insulin therapy in this patient group, based on clinical experiences and theoretical considerations, are under continuous development, and definitive results are expected in the near future.
INTRODUCTION
Epidemiology of Diabetic Kidney Disease: Why is it so Important?
During recent years, a new classification of chronic kidney disease (CKD) was introduced in the literature because this condition can be grouped as diabetic renal (DKD) and non-diabetic renal disease (NDRD), respectively. This reflects the outstanding contribution of diabetes to the development and progression of kidney damage. The prevalence of diabetes among patients on dialysis is 40–50% in developed countries.1 Challenges related to the control of carbohydrate homeostasis are more complex compared to those in diabetics without renal involvement. Indeed, even the definition of optimal glycaemic control in patients with varying degrees of renal failure is not fully clarified, and target values in individuals are significantly different.2 During recent years, the appropriate management and assessment of carbohydrate homeostasis in patients with renal disease, as well as the impact of glycaemic control on morbidity and mortality, are at the centre of scientific interest. This increased awareness could improve healthcare providers’ consciousness of and adherence to current guidelines. This review dicusses the relationship between diabetes and advanced stages of CKD, haemodialysis (HD), or peritoneal dialysis in detail.
Based on large trials performed in recent decades, good metabolic control hallmarked by HbA1c levels around 6–7% was shown to be protective against renal injury and other complications.3 However, in the presence of moderate CKD, the factors influencing mortality and a further decrease in glomerular filtration rate (GFR) change markedly compared to those in the general population or in patients with early-phase renal failure. HbA1c, for example, is not clearly associated with further progression of CKD and mortality despite being the conventional glycaemic marker.4
Worldwide morbidity due to non-communicable diseases is in general improving; to the contrary, mortality related to CKD, and the prevalence of diabetic renal damage and associated disabilities, is increasing.5 Based on prior studies (NHANES III, 10 years of follow-up; N=16,046) it is clear that mortality of patients with Type 2 diabetes mellitus (T2DM) is predominantly related to the development of renal complications.6 The assessment of cardiovascular risk in patients with renal failure is increasingly emphasised. The risk is increased partly due to traditional factors (including inappropriate control of carbohydrate homeostasis), and partly due to non-traditional and dialysis-associated factors.
Uncertainties in the Diagnosis of Diabetic Kidney Disease
The evaluation of differences between patients with DKD and those with NDRD is challenged by two major facts. The phenomenon of burnt-out diabetes, a novel subtype of the disease, was described just 5 years ago (this condition occurs when impairment of renal function results in diminished elimination of endogenous insulin, but to a level that is still sufficient for appropriate blood glucose control: therefore, antidiabetic agents can be discontinued). The epidemiological classification of this patient group is not fully clear.7 There are several factors that contribute to downward shifting of glucose homeostasis, including impaired renal and hepatic clearance of insulin, absence of gluconeogenesis in renal tissue, and, as a result of dialysis, improved insulin secretion and decreased uraemic toxicity. Patients with burnt-out diabetes should adhere to dietary recommendations. The re-initiation of any necessary antidiabetic therapies should be decided according to the results of continuous and rigorous blood glucose monitoring performed at home. Treating physicians should also perform the regular monitoring of micro and macro-angiopathic complications.
The contribution of diabetes to CKD is varied. Diabetes can be the cause of renal failure in up to one-third of cases; but it may also present as a comorbidity with CKD of other aetiologies. This presents a further challenge when the cause of renal failure is yet to be identified in patients on dialysis, and also when its impact on mortality and survival parameters is not clarified.8 The prognostic value of specific diabetic conditions, including new-onset diabetes on dialysis and after transplantation, will be better characterised in the near future.
The Significance of Glycaemic Correction at the Time of Dialysis Initiation
The mortality of diabetic patients in the period around the initiation of dialysis is particularly high. Additional to standard risk factors (heart failure, high systolic and low diastolic blood pressure, acute renal failure) a GFR value of <45 mL/min/1.73 m2 at the time of the referral to nephrologist is also a predicting factor.9 This raises the notion that mortality associated with the initiation of dialysis can be improved if cut-off GFR values of referral are increased in diabetic conditions.9 A patient with declining GFR requires meticulous attention to avoid life threatening hypoglycaemia and to prevent serious, acute cardiovascular consequences. Therefore, their medication regimen should be regularly re-assessed and adjusted according to carbohydrate intake and specific factors affecting renal health.
According to novel data, the quality of glycaemic control in the pre-dialysis phase determines the mortality after the initiation of dialysis. Analysing HbA1c and random blood glucose levels of 17,819 patients during a 1-year preceding dialysis revealed that the quality of carbohydrate homeostasis is associated with mortality during the first year of renal replacement therapy. Compared to patients with HbA1c levels of 6–7%, the mortality increased by a factor of 1.19 and 1.48 in patients with HbA1c levels of 8–9% and >9%, respectively. The occurrence of random blood glucose levels >11.1 mMol/L increased mortality by a factor of 1.34 compared to patients with glucose levels in the range of 5.5–7.0 mMol/L.10
Other observations indicate that strict glycaemic control in the early stage of diabetic nephropathy in patients with albuminuria >300 mg/day provided no benefit and did not prevent cardiovascular events.11 A meta-analysis of 29,141 diabetic patients with no or mild nephropathy revealed that good glycaemic control (HbA1c: <7% and fasting blood glucose: <6.6 mMol/L) had no significant impact on risk of mortality, development of a condition requiring dialysis, or major cardiovascular events, and provided minimum benefit regarding the risk of myocardial infarction and progression of microalbuminuria.12 The significance of the quality glycaemic control in earlier stages of DKD should be established by future studies. Previous results of large diabetological studies (UKPDS,13 DCCT,14 EDIC15) obtained one to two decades ago suggested that good glycaemic control correlated with better outcomes. Nowadays, however, the strength of this evidence is debated due to the change in the composition of global DKD population (with more elderly patients with comorbidities) and change in antidiabetic regimes. The long-term benefits of tight metabolic control on renal conditions are seemingly limited to those still not on dialysis.
MARKERS FOR QUALITY ASSESSMENT OF CARBOHYDRATE HOMEOSTATIS IN PATIENTS WITH RENAL FAILURE
Traditional Markers
Based on recent literature, the benefits of strict glycaemic control in the prevention of micro and macrovascular complications are not fully demonstrated. According to current guidelines, individualised targets should be established while considering the presence of co-morbidities, susceptibility to hypoglycaemic episodes, and predicted lifetime. This approach is of particular importance in patients with renal failure.
For the assessment of the quality of glycaemic control there are traditional and novel markers. Their role, clinical use, and justification are different in patients with renal failure compared to the general population. In older patients with renal impairment, hypoglycaemia may lead to catastrophic events including myocardial ischaemia, stroke, severe accidents, epileptiform attacks, or sudden death due to acute malignant arrhythmias.
According to some (but limited) data, variability in glucose levels may have a role in the development of DKD. One can assume that in the future, individual or professional continuous glucose monitoring (CGM) will be the standard tool for the recognition of hypoglycaemic events and the establishment of optimal therapy.16 The self-check of glucose levels alone improved glycaemic control during a 3-month period, with an efficacy comparable to that of CGM. It is likely that the patients’ increased awareness alone decreased the occurrence of hyperglycaemic phases, while the frequency of hypoglycaemic events did not increase.17 In addition, in a particular individual the estimated average of blood glucose levels based on HbA1C levels does not necessarily reflect real blood glucose levels: only CGM can provide reliable information on glycaemic control. Unfortunately, the wide use of CGM is financially limited.18
Another study demonstrated that high glycated albumin levels predict 4-year mortality in HD patients (N=1,255) better than HbA1c.19 A meta-analysis (N=3,928 from 24 studies) also revealed a stronger correlation of average blood glucose levels with glycated albumin than with HbA1C levels, and that glycated albumin levels in patients with advanced renal failure predicted cardiovascular events more accurately than HbA1c.20 Other investigators evaluated the relationship between the fasting blood glucose levels of patients with varying degrees of renal failure, and HbA1c, glycated albumin, and fructosamine levels. None of these markers were optimal for the assessment of glycaemic control in this population. Currently, however, HbA1c is still considered to be the traditional marker used for the assessment of the quality of glycaemic control; other non-traditional markers offer no benefits in prediction.
Novel Markers of Metabolism
Considering the phenomenon of metabolic memory, the lack of strong correlation between outcome and actual (short-term) glycaemic control in renal failure patients is not surprising. In general, diabetic nephropathy develops 10–15 years after the diagnosis of carbohydrate metabolic disorder, and the development of end-stage renal disease requiring dialysis lasts for several years after. However, the exact date of disease onset is not known at the time of T2DM diagnosis, and the quality of glycaemic control in a patient with advanced renal failure is not known in the long-term either; one cannot therefore establish the contribution of the quality of glycaemic control in preceding periods to the progression of renal impairment.21
Skin autofluorescence measurement is a tool that may better indicate the development of complications and risk of mortality compared to traditional markers of glycaemia. This parameter reflects the cumulative quality of glycaemic control during the preceding 5–10 years. The extent of damage to connective tissues is hallmarked clinically by osteoarthritic and musculoskeletal degenerative disorders and patient disability. 22
Presumably, metabolic disorders will be assessed in a comprehensive way at the metabolome level, which reflects also the effects of diet and gut microbiome.23 The notion of uraemic toxins is also under transition due to the recent results of metabolome research. Data suggest that diabetes has a more significant effect on some individual metabolite levels in the early phase of renal failure compared to the advanced phase.24
CHANGE OF CARBOHYDRATE METABOLISM WITH IMPAIRMENT OF GLOMERULAR FILTRATION RATE: THE IMPACT OF DIFFERENT FACTORS ON PROGRESSION
Lifestyle Factors and Diabetic Kidney Disease
Previous literature data clearly support that regular physical activity and adherence to dietary recommendations slow the onset and progression of long-term complications, including chronic renal failure. For corresponding details tailored to specific populations refer to national guidelines. The Mediterranean diet has an increasing role in the therapy of both diabetes and CKD.25 In recent years, an association between T2DM and altered gut microbiome, characterised by the reduction of micro-organisms producing short chain fatty acids from carbohydrates, has been demonstrated.26 The further alteration of microbiome during the development of nephropathy is less known. An association between oral cavity microbiome and CKD risk, as well as the blood level of certain cytokines (including IL-18), was also reported.27 During the development of CKD, the gut microbiome is skewed and protein-metabolising bacteria become more abundant: locally produced uraemic toxins are absorbed and cause systemic toxicity, aggravating renal damage (and decreasing toxin elimination via the kidneys). Therefore, dietary interventions that have an impact on the gut microbiome may improve uraemic condition and slow CKD progression.28
A further challenge when treating this population is the patients’ non-compliance and non-co-operativeness due to deterioration of cognitive skills.29
Insulin Resistance in Chronic Kidney Disease
Because of altered insulin secretion and increased insulin resistance, CKD is associated with disturbed glucose homeostasis. Novel research data identified altered gut microbiome as the common cause of diabetic and uraemic, metabolic abnormalities.30 All stages of CKD are hallmarked by insulin resistance. Several factors contributing to insulin resistance include decreased vitamin D levels, hyperparathyroidism, erythropoietin (EPO) deficiency, uraemic milieu and increased carbamylation of proteins due to high urea levels, inflammatory processes and increased oxidative stress, increased levels of cytokines, renin-angiotensin system activation, and acidosis. Standard factors of insulin resistance that are independent of renal function, including advanced age, obesity, dyslipidaemia, and hypertension, are also present. Hyperinsulinaemia via the MAPK pathway enhances vasoconstriction, cell proliferation, and cell migration, and results in alterations to the vascular wall, hence increasing the development of cardiovascular disorders.31 A possible molecular mechanism of insulin resistance is the glycation of the insulin receptor and the impairment of cells’ insulin binging capacity.32
Clinical observations support that CKD requiring dialysis is itself a risk factor of insulin resistance. Patients on a transplantation waiting list and treated with HD or peritoneal dialysis were compared to subjects from general populations who are at increased risk of T2DM. The group members were age, gender, and BMI-matched, with comparable glucose levels at baseline at the 120th minute of oral glucose tolerance test. Insulin production in the dialysed patients was significantly higher compared to that in the general population.33
The Kidney and the Carbohydrate Homeostasis
The role of the kidney in glucose homeostasis alters with the progression of renal function impairment. Under physiologic conditions, renal gluconeogenesis provides about 20–25% of glucose released into circulation: in the post-absorption phase, this figure increases up to 60%. In diabetic patients, the contribution of renal gluconeogenesis is increased further.34 The increased risk of hypoglycaemia in CKD is partly due to the absence of renal gluconeogenesis.34 Another factor contributing to increased risk associated with GFR impairment is the impaired renal elimination, as well as the more extensive or protracted effects of antidiabetic agents. Glomerular glucose filtration is also increased in diabetes. As a counterbalancing mechanism, tubular SGLT2 expression is increased, resulting in a later onset of glucosuria despite high glucose levels. With the development of renal failure, the extent of glucosuria decreases, while hyperfiltration caused by hyperglycaemia accelerates renal damage.35
Advanced Glycation End-Product Accumulation and Glycaemic Control
Both hyperglycaemia and CKD contribute to increased levels of glycation product. The increase of oxidative stress is associated with increased advanced glycation end-product (AGE) production. Renal proximal tubular cells degrade filtrated AGE products; therefore, elimination decreases as renal function impairment progresses. At the same time, AGE products accumulate in the mesangial matrix and contribute to renal damage in diabetic nephropathy.36 In diabetic patients with GFR >30 mL/min/1.73 m2 the skin autofluorescence values correlated inversely with the extent of renal impairment, and proportionally with early-stage atherosclerosis detected by ultrasound examination of carotid and femoral arteries.37 The molecular basis of these phenomena is explained by the alteration of gene transcription induced by AGE-RAGE axis activation, resulting in the acceleration of inflammatory and oxidative processes and, finally, in endothelial cell dysfunction and arteriosclerotic plaque formation. AGE products induce permanent hyperglycaemia via biochemical alterations impairing glucose utilisation, and this provides a positive feedback for the increased production of further AGE molecules. Simultaneously, they play a role in the transition of smooth cells in vascular wall to bone-producing cells, which is considered as the first step of vascular wall calcification.38
Association Between Bone Remodelling and Carbohydrate Metabolism
AGE products exert multiple effects on pathological changes of bone architecture that finally result in osteoporosis. A major effect is the increase of osteoblast FGF23 production. A pilot study found an inverse correlation between intact parathyroid hormone and skin autofluorescence values.39 Osteoblasts and adipocytes originate from a common, mesenchymal progenitor cell: during local remodelling processes there is a strong and bidirectional interaction between these two cell types. An anabolic effect of insulin is accelerated bone formation due to its binding to receptors on osteoblasts. Osteocalcin produced in bone tissue has an impact on insulin secretion and carbohydrate utilisation. Energy carrier molecules mobilised from fat stores support these processes, while the simultaneously mobilised cholecalciferol has an impact on calcium and phosphorous balance, as well as insulin secretion.40
Impact of the Correction of Anaemia on HbA1C Levels and Adverse Cardiovascular Outcomes
A study of 1,558 patients in CKD stage 3–4 revealed that HbA1C levels are only associated with mortality, risk of requiring dialysis, and cardiovascular events only in patients with haemoglobin levels >100 g/L: no correlation was observed in anaemic patients. HbA1C levels are influenced by a number of factors, including EPO therapy and conditions resulting in anaemia such as malnutrition or chronic inflammatory disorders.41 HbA1C levels at the same glycaemic control are increased in the presence of iron deficiency,42 while decreased upon EPO therapy.43 Early observations from the Glycemic Indices in Dialysis Evaluation (GIDE) study indicate an inverse association between EPO therapy and HbA1C levels.44 EPO therapy didn’t show correlation with other alternative glycaemic markers, so lower HbA1C levels might be considered as a result of accelerated erythropoiesis, and therefore, this marker is not suitable for the characterisation of glycaemic quality.43 EPO has pleiotropic, anti-apoptotic, and immune-modulatory activity. Presumably, it modulates glucose homeostasis through several means in addition to stimulated haematopoiesis.45
Beta Cell Dysfunction in Chronic Kidney Disease
Simultaneous to the impairment of renal function, uraemic toxin levels are also increasing: these toxins contribute to insulin resistance. In later stages, this process is accompanied by the dysfunction of beta-cells causing defective insulin secretion. Further factors accelerating beta-cell damage are acidosis, dyslipidaemia, hyperuricaemia, vitamin D deficiency, disturbed bone metabolism, and secondary hyperparathyroidism. However, results obtained so far are not unequivocal regarding the relationship between GFR impairment and the development of diabetes due to beta-cell dysfunction.46 A large study (N=1,337,452; median duration of follow-up 4.9 years) demonstrated that, initially, a high urea level was an independent risk factor for the later development of diabetes.47
HAEMODIALYSIS AND GLYCAEMIC CONTROL
Common Features of the Two Dialysis Modalities
Depending on the glucose levels of the dialysis solution, the body loses or gains glucose during dialysis sessions. Both modalities have an impact on the metabolism of lipids, amino acids, and carbohydrates. Glucose entering from dialysis solution into the body potentiates insulin resistance and hyperinsulinaemia caused by the uraemic condition. The use of glucose-free solutions, on the other hand, is a risk factor of severe hypoglycaemic events in patients treated with antidiabetic agents. The permanent or intermittent parenteral glucose load has wide-ranging metabolic effects: for instance, via acceleration of oxidative stress, it contributes to increased glycation and has an adverse effect on cardiovascular outcomes.48 Proinflammatory processes are also activated and accelerated at the time of the initiation of dialysis, depending on the extent of biocompatibility and pyrogen content of the dialysing agent. However, the increase of inflammatory marker levels is partly due to the failure of their renal elimination.
Data on the incidence of diabetes following the initiation of dialysis are still equivocal. A study from the Far East reported that during 13 years of follow-up the risk of novel diabetes is decreased (HR: 0.49) in patients subjected to regular HD compared to controls (8,912 patients on HD, 2,092 patients on peritoneal dialysis, and 136 of controls). Of note, the decrease in risk was detected only in patients with HD; the incidence of diabetes in the peritoneal dialysis group was comparable to control patients, indicating that diabetes incidence was higher in patients treated on peritoneal dialysis than in those on HD (15.98 versus 8.69 case/1,000 patient years).49
Effects of Technical Parameters on Carbohydrate Metabolism in Haemodialysis
In HD patients, CGM revealed significant changes in carbohydrate metabolism depending on the day of dialysis session: average blood glucose levels and deviation of glucose levels were higher on days of dialysis than on those without sessions. This indicated a periodic change in insulin requirement.50 In a 12-month study, the efficient removal of uraemic toxins with high-volume haemodiafiltration significantly improved malnutrition, the risk of protein malnutrition, and CRP values reflecting inflammatory processes.51 AGE values and large molecular weight uraemic toxins are also removed from the body during dialysis sessions. In a small study, skin autofluorescence values decreased significantly (5.2%; p=0.02) 1 week after switching the patients to glucose-free dialysis solutions. Plasma autofluorescence values decreased after each HD session, probably due to the reduction of protein-bound fraction: the values reached their nadir after 2 weeks of therapy (p<0.05).52
Meals during HD increase the risk of hypotensive episodes due to redistribution of circulation accompanying digestion. This adversely modulates blood flow and impairs the efficacy of dialysis. However, even in the presence of glucose-containing dialysis solution (with a glucose level of 8.33 mMol/L) the metabolic state of diabetic patients is skewed into a state resembling that of fasting. During dialysis, the lactate, pyruvate, and alanine levels decreased, while 3-hydroxy-butirate and ketone body levels increased in diabetic patients. In addition, plasma insulin levels decreased due to filtration and adsorption during HD; diabetic patients with a deficient capacity to produce endogenous insulin were not able to compensate this phenomenon. Due to impaired glycolysis, this results in alternative mobilisation of energy sources, in imbalance of fat and protein metabolism into catabolic state, and increased gluconeogenesis. Similar alterations were not observed in HD patients without diabetes. Therefore, the administration of exogenous insulin at the end of the HD session should be individually considered and assessed.53
The Phenomenon of Glycaemic Disarray
The development of hypoglycaemic events in HD patients is the result of a complex array of factors that includes decreased appetite, failure of renal gluconeogenesis, impaired renal insulin clearance, glucose entering out of the body into the dialysing solution, the use of glucose-free dialysis solution, and increased erythrocyte glucose uptake during dialysis session. Therefore, the dosage of insulin and oral agents capable of inducing hypoglycaemia should be carefully determined, especially when both the efficacy and duration of these medicines increase. Several hours after HD, however, paradox rebound hyperglycaemia occurs, probably as a result of hormones counterbalancing insulin effects (similarly to that seen with Somogyi’s effect). The insulin itself, along with peptide-type substances which impact on insulin secretion and effect, are removed during dialysis, while some membranes bind circulating insulin.54 HD diabetic patients require individualised insulin dosing regimes for HD-days and non-HD days. The majority require less exogenous insulin during the post-dialytic period, while some require additional doses in the immediate post-HD hours. The modifications should be assessed according to blood glucose results in each patient.
Clinical Specificities in Haemodialysis
Lifestyle modification, including regular physical activity, is part of routine recommendations for HD patients. Some experts suggest controlled activity during dialysis sessions, while others prefer exercises on dialysis-free days that are recorded in a diary. In addition to the ageing of patients requiring dialysis, specific dietary considerations and cognitive impairment require individual adaptation of dietary modifications. Sarcopenia, (i.e., the severe decrease of active muscular mass) is increasingly frequent among the dialysed patients: the prevalence of the so-called frailty condition may be up to 60%.55
Oxidative Markers in Haemodialysed Patients
An American study analysed a large number of patients (N=16,387) and reported a correlation between 2-year survival of HD patients and HbA1C levels. In patients with HbA1C levels >8.5%, the cardiovascular mortality was 18% higher compared to that in patients with HbA1c levels <6.5%. The increased mortality was due to a higher risk of myocardial infarction; stroke, peripheral vascular disease, and all-cause mortality were comparable in the two groups.56
The association between HbA1c levels and mortality is characterised by a U-shaped curve. The mortality was the lowest in patients with HbA1c 6–7% in the JDOPPS study; HbA1c <5% and 5–6% were 1.56 (95% CI: 1.05–2.33) and 1.26 (95% CI: 0.92–1.71), respectively.57
In T2DM patients on HD, both random plasma glucose levels and glycated albumin levels correlated with xanthine-oxidoreductase activity; therefore, this enzyme may be partially responsible for the acceleration of oxidative processes in poorly controlled diabetic patients.58 In the general population the plasma xanthine-oxidoreductase activity correlated with BMI, smoking, uric acid and triglyceride levels, and insulin resistance index. The increased activity of the enzyme is accompanied by the generation of superoxide and other reactive free radicals. Therefore, this marker can be considered as the general biomarker of metabolic disorders.59
PERITONEAL DIALYSIS AND GLYCAEMIC CONTROL
Theoretical Considerations
Obligatory glucose absorption inherent with peritoneal dialysis increases cardiovascular risk. Daily glucose load ranges between 50–180 g depending on the regime used and transport characteristics. In diabetic patients, the blood glucose control should be intensified and individualised depending on hyperinsulinaemia and obesity. The benefits of peritoneal dialysis that are attributed to steady toxin and fluid removal, and maintenance of residual renal functions, begin to disappear if metabolic control is of not appropriate quality.60 In peritoneal dialysing solutions containing high levels of glucose, so-called glucose degradation products (GDP) are formed during the sterilisation processes on high temperature. Characteristic GDP are methylglyoxal, acetaldehyde, formaldehyde, and 3-deoxyglucosone. These agents induce AGE production. Methylglyoxal also has a direct inhibitory effect on the insulin signalling pathway and increases insulin resistance. Actual guidelines on peritoneal dialysis, therefore, include the minimisation of glucose load during sessions and the use of glucose free solutions.61
Effects of Technical Parameters on Carbohydrate Metabolism in Peritoneal Dialysis
The systemic glucose exposure is increased by peritoneal dialysis. A study enrolled patients who are treated with peritoneal dialysis for at least half a year, but are not suffering in disorders of carbohydrate metabolism. Results indicated that in this peculiar population random plasma glucose levels correlated with daily intraperitoneal glucose exposure.62 To avoid local and systemic damage caused by increased glucose load, glucose-free solutions and solutions with low GDP content are increasingly used, particularly when treating diabetics. Currently, there are no reliable data about the benefits of biocompatible dialysis solutions and icodextrin-containing, glucose-free polysaccharide solutions on technical survival and clinical outcomes.
Compared to patients using solely glucose-based solutions, patients who use icodextrin-based solution for long daytime dwell exhibited improved glycaemic control based on fasting glucose levels, daily cumulative insulin dose, and HbA1c.63 In general, peritoneal dialysis regimes contain icodextrin solutions just once a day due to financial limitations and the fear of possible side effects (including allergic events, aseptic peritonitis, extensive ultrafiltration, and interference of absorbed maltose with some methods used for glucose measurements). However, patients who require strict glucose restriction and intensified ultrafiltration are increasingly treated with icodextrin twice a day or with a bimodal peritoneal solution containing both icodextrin and glucose.64
Clinical Specialities in Peritoneal Dialysis
The glycaemic control of diabetic patients with nephropathy depends in general on patients’ adherence. Regular self-checks of glucose levels are of particular importance as therapy is based on actual blood glucose levels. Long-term glycaemic markers, however, provide less support to manage therapy, albeit patients with very low or those with very high blood glucose levels are at high risk of morbidity. In patients on long-term peritoneal dialysis, the gradual decrease of residual renal function and urinary output require the introduction of solutions with high glucose content in order to maintain optimal hydration. Although, in the short-term dwell time or the number of exchanges with the same low concentration glucose solution can be increased. This results in increased glucose exposure; therefore, the strategy of glycaemic control should be reassessed.65
Permanently high glucose exposure causes significant changes of peritoneal surface and leads its remodelling in a still unclarified way. Finally, the physiologic anti-adhesive properties are lost. Encapsulating peritoneal sclerosis is a rare, but dangerous complication of peritoneal dialysis; histologically it resembles diabetic micro-angiopathy. The most dominant risk factor is the length of period since the initiation of peritoneal dialysis supports the possible contribution of cumulated peritoneal glucose exposure. The episodes of peritonitis and the development of quick transporter character are further risk factors of encapsulating peritoneal sclerosis and indicate the pathogenic role of locally high glucose exposure.66
Epidemiological Data in Peritoneal Dialysis
In diabetes, the benefits of peritoneal dialysis compared to HD in terms of survival following the initiation of dialysis sessions were demonstrated only in those patients who reached optimal glycaemic control (HbA1c: <8%). If glycaemic control was not optimal, there was no difference in mortality between peritoneal and HD.67 There are several unclarified issues, however, regarding the importance of optimal glycaemic control in dialysed patients. It is not known whether the impact of optimal glycaemic control on prevention or delay of complications is so significant in nephropathy as in patients without renal failure. There is still no consensus even on optimal therapeutic targets either. The question of whether peritoneal dialysis should be the first-choice modality in diabetic patients can be answered after the extensive statistical analysis of large number of clinical data from patients initiating dialysis.68
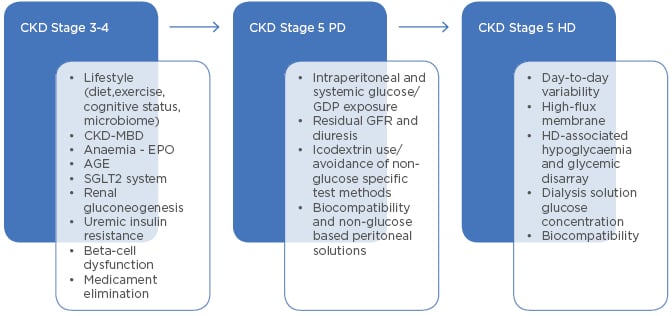
Figure 1: Main factors of glycaemic change in different states of renal failure.
AGE: advanced glycation end-product; CKD-MBD: chronic kidney disease-mineral and bone disorder; EPO: erythropoietin (therapy); GDP: glucose degradation products; GFR: glomerular filtration rate; HD: haemodialysis; PD: peritoneal dialysis; SGLT2: sodium-glucose co-transporter-2.
CONCLUSIONS
Factors that are associated with CKD and related to the modality of dialysis (Figure 1) should be considered sufficiently when planning and controlling therapy of diabetic patients with renal failure. DKD raises several specific questions regarding the glycaemic control of affected patients. The optimal approach should be assessed individually in each patient, comprehensively considering the relevant technical parameters and clinical data. Targets may differ depending on whether the patient is in good general condition and waiting for transplantation, or if the patient is multi-morbid, disabled, and aged. In the first scenario, carbohydrate metabolism along with cardiovascular risk factors should be strictly controlled; in the second case, however, the major goal is the short-term improvement of overall life quality.
Carbohydrate metabolism of patients with advanced renal failure is more labile compared to that of the general diabetic population. It is affected by several additional factors related to renal failure itself and applied therapy, and subject to this review. Regarding antidiabetic control, the major therapeutic goals in patients on HD is the alleviation of glycaemic fluctuation during the postdialytic phase. The periodicity in antidiabetic regimes on HD and non-HD days is a preferable tool for individualised therapy. In patients on peritoneal dialysis, the adverse impact of glucose originated from standard peritoneal dialysis solutions should be counterbalanced.








