Meeting Summary
After a welcome by Paolo Antonio Grossi, the appointed chair by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Department of Medicine & Surgery, University of Insubria, Varese, Italy; Ann-Brit Eg Hansen, Department of Infectious Diseases, Copenhagen University Hospital – Amager and Hvidovre, Denmark and Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark, summarised the objectives of the meeting and introduced the speakers. In their opening presentation, Tobias Welte, Department of Pulmonary and Infectious Diseases, Hannover University School of Medicine, Germany, described how the COVID-19 pandemic has evolved, in terms of variants, mortality rates, vaccinations, immunity, and antivirals. Welte then presented a hypothetical case study to illustrate how older patients with comorbidities can initially have mild symptoms, but may then deteriorate and require hospitalisation. Stephen Thomas, State University of New York Upstate Medical University, Syracuse, USA, then described the efficacy and potential side effects of the BNT162b2 (Pfizer–BioNTech, New York City, USA, and Mainz, Germany, respectively) vaccine, and the need for, and benefits of, booster doses. Thomas also described the added benefits of the newer bivalent vaccines in a world where COVID-19 is constantly mutating. Marta Boffito, HIV, Sexual and Gender Health, Dermatology, Chelsea and Westminster Hospital NHS Foundation Trust, Imperial College London, UK, then outlined various factors that increase the risk of progression to severe COVID-19 disease, including older age, immunocompromised status, and underlying health conditions (e.g., obesity, hypertension, heart disease, and chronic kidney disease [CKD]). Such patients can benefit from antiviral medications such as nirmatrelvir/ritonavir, although potential drug–drug interactions must be considered. Roger Paredes, Department of Infectious Diseases and IrsiCaixa AIDS Research Institute, Hospital Universitari Germans Trias i Pujol, Badalona, Catalonia, Spain, and Center for Global Health and Diseases, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA, revisited the case study to highlight the importance of early COVID-19 diagnosis among high-risk patients to enable the use of nirmatrelvir/ritonavir, which is only approved within 5 days of symptom onset for non-hospitalised adults at increased risk of progression to severe COVID-19. Paredes went on to discuss antiviral treatments in more detail, describing a randomised controlled trial (EPIC-HR) and two large real-world studies that showed that nirmatrelvir/ritonavir could significantly reduce the risk of hospitalisation and death due to COVID-19 among high-risk patients. To conclude, Hansen highlighted the importance of regular updates to COVID-19 management guidelines, given the ongoing and evolving nature of COVID-19, as well as the importance of identifying high-risk patients early in their disease course to enable the use of nirmatrelvir/ritonavir.
A COVID-19 Perfect Storm: Reviewing a Clinical Challenge
Tobias Welte
Since COVID-19 was first identified in late 2019 in Wuhan, China, key variants have included the Alpha and Delta variants.1 However, by the end of 2022, most cases were Omicron,2 which is more transmissible but less deadly. There are multiple variants of Omicron, of which some are termed “variants of concern.” Most recently, XBB.1.5 was dominant in Western Europe, and this has increased transmissibility.3
Despite the reduced mortality risk of COVID-19, the high incidence means that there are still patients with COVID-19 who need hospital treatment and some who die, especially high-risk patients. Fortunately, low-risk patient groups (i.e., younger people without comorbidities) have good immunogenicity due to high vaccination rates4 and exposure to infection. However, vaccinations for low-risk groups have reduced dramatically, and due to waning immunity and lower infection rates over the summer, immunogenicity is falling, the impact of which is not yet known.
The World Health Organization (WHO) recently acknowledged COVID-19 as an ongoing public health emergency.5 Recommendations include: 100% vaccination rates for high-priority groups; to increase uptake and ensure availability of medical countermeasures; improved surveillance; and continued engagement with communities.5
Welte presented a hypothetical case study of a 70-year-old female who had a positive home COVID-19 test 6 days after symptom onset (Figure 1). They were receiving amlodipine for hypertension and had mild renal impairment. They had received two primary vaccine doses, and a booster 8 months prior to contracting the virus. The patient’s COVID-19 symptoms up to Day 6 were mild.
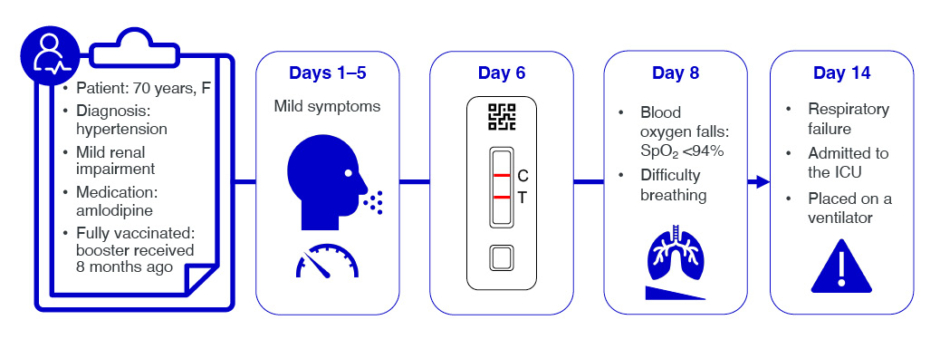
Figure 1: Hypothetical COVID-19 case timeline.
C: control; F: female; ICU: intensive care unit; SpO2: oxygen saturation; T: test.
Typical primary symptoms of COVID-19 include headache, fatigue, shortness of breath, cough, loss of sense of taste or smell, muscle aches, nausea, and diarrhoea.6 These symptoms typically last for approximately 1 week, followed by a decrease in viral load.7 For many patients, their initial host immune response controls the infection and they then recover. However, in some patients, symptoms can become more severe during a second immune response phase, when infection can result in COVID-19 pneumonia.8 Some of these patients go on to have systemic hyperinflammation, which can damage the lung and other organs.8
Approximately 25–30% of people with COVID-19 are asymptomatic.9,10 At the other end of the spectrum, approximately 15% are admitted to hospital, and 5% require care in an intensive care unit (ICU).11 Mechanical ventilation is required by approximately 4% of patients who are hospitalised, increasing to 11% of those with severe acute respiratory infections.12 In-hospital mortality is approximately 6%, but increases to approximately 16% in patients with severe acute respiratory infection.12
If antiviral medications are to be given, this needs to be done early in the disease course, while the viral load is still high. However, the case patient only tested on Day 6 (Figure 1). By Day 8, they had deteriorated, with signs of COVID-19 pneumonia, O2 saturation <94%, and breathing difficulties. The patient was admitted to hospital, where they were given O2 support and corticosteroids. A CT scan revealed bilateral parenchymal opacities in >50% of the total chest area. The patient deteriorated, went into acute renal failure, which is a strong predictor of mortality, and ultimately required invasive mechanical ventilation in the ICU.
Currently, immunocompromised patients (i.e., transplant recipients and patients with cancer or autoimmune disease) are considered to be at the highest risk from COVID-19. However, older adults with comorbidities are also at increased risk of severe COVID-19 disease, and ways to manage such patients are discussed below.
Expert COVID-19 Consultation: Vaccination for the Prevention of COVID-19
Stephen Thomas
The recommended primary vaccination course with the BNT162b2 vaccine is: three doses ×3 µg/dose for children aged 6 months–4 years; two doses ×10/dose µg for children aged 5–11 years (three doses if severely immunocompromised); and two doses ×30 µg/doses for children aged ≥12 years and adults (three doses if severely immunocompromised).
As with all vaccines, there is the potential for adverse reactions, including hypersensitivity, anaphylaxis, myocarditis, and pericarditis.13 The risks of hypersensitivity and anaphylaxis are similar to other routinely used vaccines, but appropriate treatments should be available at vaccination centres. Myocarditis and pericarditis are very rare reactions, occurring at a rate of less than one case per 10,000 vaccinations; they have been observed more often in young males than other demographic groups, and after a second dose.13,14 Myocarditis and pericarditis generally occur within 14 days after vaccination.13,14 Healthcare providers should, therefore, be alert to the signs and symptoms of myocarditis and pericarditis, namely acute and persisting chest pain, shortness of breath, or palpitations.
Among individuals aged ≥16 years, BNT162b2 vaccine efficacy in preventing first COVID-19 occurrence was >90% at the time of the study, when Wuhan/Wild type and Alpha variants were the predominant circulating strains.13 Among individuals aged 5–11 years and 12–15 years, a similar safety profile and vaccine efficacy was observed for BNT162b2. Immunobridging criteria were also met in children aged 5–11 years and adolescents aged 12–15 years at 1 month after dose 2, when compared with young adults aged 16–25 years. Among younger children (6 months–4 years), BNT162b2 resulted in >70% vaccine efficacy in preventing first COVID-19 occurrence when the Omicron variant of severe acute respiratory syndrome coronavirus 2 (BA.2) was the predominant circulating strain. Immunobridging criteria were also met in children aged 6 months–4 years at 1 month after dose 3, when compared with young adults aged 16–25 years.13
Later, a small Phase I study showed that immunogenicity waned over time after the second dose of BNT162b2, and also that protection against the Beta variant was lower than against the Wild type variant.15 However, a booster dose among adults aged 18–55 and 65–85 years resulted in increased immunity against both strains.15 A larger, Phase II study further supported the need for, and potential benefit of, a booster dose.13
In a large study, individuals aged ≥16 years who had received two doses of BNT162b2 were randomised 1:1 to a booster dose of BNT162b2 or placebo.16 The boosters were administered a median of 11 months after the second dose, and the main strain circulating at the time of the study was Delta. After a median follow-up of 2.5 months, only five individuals who had received a booster dose of BNT162b2 developed COVID-19, compared with 109 in the placebo group (giving a vaccine efficacy of 96%), and there were no new safety signals.16
After these studies, the Omicron BA.1 variant became dominant in early 2022.17 It became apparent that the components of the vaccine needed to be better matched to the circulating strains, hence a bivalent vaccine that contained the original Wuhan component plus BA.1 was developed.13 This had a similar safety profile to the original vaccine, and induced neutralising antibodies to both strains. In the summer of 2022, Omicron BA.5 and BA.4 appeared,17 and a bivalent vaccine containing the original strain and BA.4/5 was developed.13
Among individuals who had received three doses of the original BNT162b2 vaccine, a booster dose of the bivalent BNT162b2 original/Omicron BA.4/5 vaccine elicited similar neutralising antibody titres against the original strain as a booster dose of the original vaccine, but higher neutralising antibody titres against various other Omicron sublineages, such as BA.4, BA.5, BA.4.6, BQ.1.1, XBB.1, and BA.2.75.2.18
A large, retrospective study of individuals aged ≥12 years with a positive COVID-19 test result was undertaken at a time when Omicron BA.4.6, BA.5, BQ.1, and BQ.1.1 sublineages were predominant.19 The bivalent vaccines were shown to offer better protection against severe disease than the original vaccine (efficacy against hospitalisation: 59% versus 25%; efficacy against hospitalisation or death: 62% versus 25%), with similar results among adults (≥18 years), older adults (≥65 years), and those without previous COVID-19 infection.19
In summary, Thomas highlighted the need to match vaccine components to circulating COVID-19 variants.
Identifying Patients at Risk of Severe Disease Progression
Marta Boffito
Boffito recapped the case study presented earlier of the 70-year-old female with hypertension and mild renal impairment, who only tested for COVID-19 after 6 days of mild symptoms, and then progressed to severe disease (Figure 1). Boffito highlighted the importance of identifying patients at risk of severe disease progression so that testing is done in a timely manner, and treatments can be administered earlier.
Risk factors for severe COVID-19 include: older age; immunocompromised status, due to disease or immunosuppressants; underlying health conditions; and current or recent pregnancy.20-22 According to data from the Centers for Disease Control and Prevention (CDC), the risk of COVID-19 infection is similar across age groups, but the risk of hospitalisation increases five-fold among those aged 65–74 years and 15-fold among those aged ≥85 years, compared with individuals aged 18–29 years.23 The risk of death increases even more substantially, being 65-fold and 360-fold higher among people aged 65–74 and ≥85 years, respectively, versus 18–29 years.23
Patients who are immunocompromised are also at increased risk of severe COVID-19, including:24 haematopoietic stem cell transplant recipients; solid organ transplant recipients taking immunosuppressive therapy; patients undergoing treatment for solid or haematological cancers; patients with moderate or severe primary immunodeficiency; patients with advanced or untreated HIV infection; and patients taking immunosuppressive or immunomodulatory treatments.
Underlying health conditions that can increase the risk of severe COVID-19 include:25 diabetes, obesity, and hypertension; ischaemic heart disease and history of heart failure; solid organ tumours; chronic respiratory disease and chronic kidney disease; and neurological conditions.
Boffito described several studies that have looked at the risk associated with various underlying heath conditions in more depth. A systematic review has shown that patients with chronic obstructive pulmonary disease (COPD) who develop COVID-19 are at a 1.4-fold increased risk of hospitalisation and death compared with people without COPD.26 Among patients with CKD, the risk of death from COVID-19 was approximately doubled after adjustment for confounding variables.27 In a small study of 41 patients who had received a kidney or kidney/pancreas transplant and developed COVID-19, 56% required hospitalisation, even though all but one had received two or more doses of a COVID-19 vaccine.28
Indeed, several studies have demonstrated a residual vulnerability in patients with underlying health conditions, even after vaccination. A retrospective cohort study of adults who developed COVID-19 during the Omicron era, despite having received three or more COVID-19 vaccine doses, showed that significant risk factors for hospitalisation were: hypertension (2.3-fold increased risk); CKD (2.2-fold); myocardial infarction/heart failure (2.2-fold); age (1.2-fold per 10-year increase); and time since last vaccination dose,29 consistent with the observed waning of immunity described by Thomas. A prospective cohort study of adults in the UK who had received one or two vaccine doses produced similar results, and identified significant risk factors for severe COVID-19, including: CKD (increasing from 1.3-fold increased risk of hospitalisation for patients with Stage 3 CKD, up to 12.8-fold for kidney transplant recipients); bone marrow or solid organ transplantation (6.8-fold); blood cancer (1.9-fold); diabetes (1.3–1.8-fold, depending on glycated haemoglobin levels); atrial fibrillation (1.4-fold); congestive cardiac failure (1.4-fold); COPD (1.3-fold); coronary heart disease (1.3-fold); and stroke (1.2-fold).30 This study also showed that the risk of COVID-19-related hospitalisation was reduced by 79% after two versus one dose of vaccine,30 highlighting the importance of multiple vaccine doses. However, this study did not look at the impact of booster doses on residual risk of severe disease in patients with underlying conditions.
Similarly, in patients who are immunocompromised, a healthcare database study found that the BNT162b2 vaccine was effective in individuals who were taking immunosuppressants (e.g., disease-modifying antirheumatic drugs and glucocorticoids), but that such patients were at higher risk of COVID-19 infection and hospitalisation than immunocompetent individuals.31
Together, these studies highlight a range of comorbidities that physicians and patients need to be aware of to facilitate timely intervention. Boffito revisited the case study, in which a patient with hypertension and mild renal failure, in whom COVID-19 was not confirmed until Day 6, was hospitalised and admitted to ICU, demonstrating the potential serious consequences of failing to act promptly in patients with risk factors.
The evidence for intervention in the form of antiviral treatment was discussed by Parades and is outlined in the next section, but Boffito summarised the latest WHO guidelines for antiviral treatments for COVID-19 in people with the highest risk of hospitalisation, including:32 a strong recommendation for nirmatrelvir/ritonavir; weak or conditional recommendations for molnupiravir and remdesivir; and a strong recommendation against monoclonal antibody treatment with sotrovimab.
When prescribing nirmatrelvir/ritonavir, potential drug–drug interactions must be considered.33,34 Although nirmatrelvir/ritonavir can be given with various other medications, there are some contraindications, so these should be checked.33,34
Overall, it should be recognised that the speed of the development of COVID-19 vaccines and treatments has been unprecedented.35 However, it is important to identify and treat high-risk patients early in their disease course, which can also help to reduce transmission.36 Treatment with oral antivirals is manageable for various high-risk patient groups,34 and although knowledge of drug–drug interactions is key, this potential problem should not stop most patients from receiving oral antivirals, as the majority of drug–drug interactions are manageable.33,34
What is the Evidence for Antiviral Therapy?
Roger Paredes
Going back to the case study (Figure 1), the 70-year-old female patient with hypertension could potentially have benefitted from nirmatrelvir/ritonavir, had their COVID-19 been diagnosed earlier. Given the patient’s age and comorbidities, particularly hypertension, they would have been considered to be at increased risk of severe COVID-19.25,37 However, nirmatrelvir/ritonavir should be given as soon as possible after the onset of COVID-19 symptoms, and within 5 days.33
COVID-19 symptoms can appear 2–14 days after exposure;6 and viral loads are highest around the time of symptom onset and decline thereafter.38-40 As cases are unlikely to be detected before symptom onset, detection as soon as possible after symptom onset is therefore important in high-risk patients, and this can be achieved using various diagnostic tests.38 High-risk patients can progress quickly to severe disease, highlighting the need for rapid therapeutic intervention.8,36 Modelling studies using viral shedding duration as a surrogate for COVID-19 severity suggest that early testing and treating are a promising approach for reducing the risk of severe disease.41
Antivirals can target different parts of the viral replication mechanism, including:42-44 binding to the angiotensin-converting enzyme 2 receptor (e.g., neutralising monoclonal antibodies, convalescent plasma, and vaccines); viral entry or exit (e.g., human protease inhibitors); proteolysis (e.g., protease inhibitors); and RNA replication (e.g., RNA polymerase inhibitors).
The approved indication for nirmatrelvir/ritonavir in the European Union (EU) is for the treatment of COVID-19 in “adults who do not require supplemental O2 and who are at increased risk for progressing to severe COVID-19”.33 It is, therefore, for patients who have not yet been hospitalised, although the 5-day course should be finished if a patient becomes hospitalised.33 The recommended dose is 300 mg nirmatrelvir plus 100 mg ritonavir every 12 hours for 5 days.33 This dose is also suitable for patients with mild-to-moderate hepatic impairment or mild renal impairment, but for those with moderate renal impairment, the dose of nirmatrelvir should be reduced to 150 mg.33 Nirmatrelvir/ritonavir is not suitable for patients with severe hepatic or renal impairment.33 Other contraindications include hypersensitivity to the ingredients, and co-administration with certain drugs that are potent CYP3A inducers, or that are highly dependent on CYP3A for clearance (for full details, please refer to the summary of product characteristics).33
Going back to the case patient (Figure 1), nirmatrelvir/ritonavir would have been appropriate for this patient if they had tested positive for COVID-19 within 5 days of symptom onset. However, as the patient was taking amlodipine, they would have required careful monitoring of therapeutic and adverse effects.33
Key evidence to support the use of nirmatrelvir/ritonavir comes from a large, Phase II/III, randomised, double-blind, placebo-controlled trial of non-hospitalised, unvaccinated, symptomatic adults with a first COVID-19 infection who were at high risk for progression to severe disease.45 The EPIC-HR study was performed at a time when the Delta variant was dominant.46 In total, 2,246 patients were randomised to nirmatrelvir/ritonavir 300/100 mg or placebo every 12 hours for 5 days.45 The primary endpoint was the proportion of patients with COVID-19-related hospitalisation or death from any cause through Day 28, among patients who started treatment within 3 days of symptom onset. A key secondary endpoint was the same outcome among patients who started treatment within 5 days of symptom onset.45 The most common risk factors for severe COVID-19 among the patients in the EPIC-HR study population were: BMI ≥25 kg/m2 (80.5%), smoking (39.0%), and hypertension (32.9%); and 61.0% of patients had two or more risk factors.45
Among 697 patients who received nirmatrelvir/ritonavir within 3 days of symptom onset, 0.7% had COVID-19-related hospitalisation or death by Day 28 compared with 6.5% of 682 patients who received placebo, giving a relative risk reduction of 88.9% (p<0.001).33,45 Among 2,085 patients who received treatment within 5 days of symptom onset, results were similar (0.9% versus 6.3%; relative risk reduction: 86.3%; p<0.0001).33 There were 12 deaths, all of which occurred in the placebo arm.33,45 The most common adverse events in the nirmatrelvir/ritonavir arm were dysgeusia (5.6%) and diarrhoea (3.1%).33,45
It should be noted, however, that EPIC-HR included unvaccinated patients with a first COVID-19 infection, whereas nowadays, most people have been vaccinated and/or infected. Although randomised controlled trials provide high-quality evidence and are the basis for regulatory approval, real-world evidence has various strengths, including: larger and more diverse patient populations, being more reflective of clinical practice, and the potential for longer follow-up.47,48 However, limitations of real-world evidence include selection bias, confounding, and variable data quality.49
A high-quality real-world study of nirmatrelvir/ritonavir has recently been published.50 This Pfizer-sponsored study was conducted in California, USA, and included non-hospitalised patients aged ≥12 years with COVID-19 during April–October 2022 (i.e., the Omicron era).50 The study was conducted within the Kaiser Permanente Southern California healthcare system.50 Patients who had been prescribed nirmatrelvir/ritonavir were considered to be exposed from the dispensing date.50 A total of 7,274 patients who had been prescribed nirmatrelvir/ritonavir were matched on date, age, sex, BMI, clinical status, vaccination history, comorbidities, and healthcare seeking during the previous year to 126,152 controls who were not prescribed nirmatrelvir/ritonavir.50 Most patients had received two or more COVID-19 vaccine doses (93.9% of those prescribed nirmatrelvir/ritonavir and 85.1% of controls).50
After adjustment for differences in risk status, receipt of nirmatrelvir/ritonavir within 5 days of symptom onset was associated with an estimated effectiveness for preventing all-cause hospitalisation and death within 30 days of a positive COVID-19 test of 79.6% (p=0.008), while receipt at any time after symptom onset was associated with an estimated effectiveness of 53.6% (p=0.031).50 For the prevention of all-cause ICU admission, mechanical ventilation, or death within 60 days, the estimated effectiveness rates were 89.2% (p=0.075) if given within 5 days, and 84.1% (p=0.027) if given at any time.50 Limitations included: incomplete data, potential misclassification of immunity, unmeasured confounding, low event rates, and the use of all-cause endpoints.50 Also, some patients may not have taken the drug as prescribed.50 However, nirmatrelvir/ritonavir was found to help prevent hospitalisation and death in a highly vaccinated population, and early treatment was more beneficial than later treatment.
Another high-quality real-world study of nirmatrelvir/ritonavir was conducted independently in the USA.51 This study included non-hospitalised, vaccinated adults who developed COVID-19 ≥1 month after vaccination.51 A total of 1,130 patients who were prescribed nirmatrelvir/ritonavir were propensity score matched to 1,130 individuals who were not. Common comorbidities included hyperlipidaemia (57.5% versus 58.5%) and hypertension (52.2% versus 51.2%). Nirmatrelvir/ritonavir reduced the risk of all-cause emergency room visit, hospitalisation, or death at 30 days by 45% (p<0.005). Limitations include potential unmeasured confounding, retrospective data, and the use of all-cause endpoints. Nonetheless, a significant benefit of nirmatrelvir/ritonavir was confirmed.
Parades emphasised that antiviral treatment should not be considered a substitute for vaccination, as vaccination remains the primary strategy for protecting patients against severe COVID-19. However, antivirals are a valuable additional tool for reducing the residual risk of severe disease in vulnerable groups, such as the immunocompromised or patients with high comorbidities, particularly when started early.
Summary and Close
Ann-Brit Eg Hansen
Hansen concluded the meeting by saying that COVID-19 management guidelines need to be updated regularly by infectious disease specialists because the COVID-19 situation is ongoing and constantly evolving. The engagement of primary healthcare providers is also vital, to help identify patients who are at high risk of progression to severe COVID-19. This has to be done early in their COVID-19 disease course so that nirmatrelvir/ritonavir can be prescribed.