Meeting Summary
During the COVID-19 pandemic, the circulation of influenza and other respiratory viruses was temporarily interrupted. However, these viruses are back in circulation, and the co-circulation of influenza, SARS-CoV-2, respiratory syncytial virus (RSV), and other viruses leads to a significant annual burden, especially in extreme age groups. This article summarises the key insights on influenza and other respiratory viruses from oral and poster presentations delivered by global experts at the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) Global 2024 congress held in Barcelona, Spain, in April 2024. It discusses the impact of COVID-19 on influenza virus circulation and the implications for future vaccination strategies, the importance of surveillance and diagnostics for assessing disease burden and for detection of early variants of pandemic concern, and the broader burden of influenza, including heart attacks and pneumonia. The article also discusses the importance of conventional randomised controlled trials (RCT), and the need for more efficient RCTs to improve data quality for an accurate assessment of vaccine safety and performance to inform policy decisions and contribute to global health outcomes.
Introduction
Respiratory viruses such as influenza, SARS-CoV-2, and RSV can lead to severe consequences and complications, especially among the elderly and those with risk factors such as lung, metabolic, and cardiovascular diseases.1 These infections may result in functional decline, disability, a decreased quality of life, and an increased risk of mortality, especially in older populations.1,2 Seasonal infections can lead to a reduced workforce and add pressure on healthcare systems annually.2 Occasionally, pandemics that are caused by viruses that have a wide range of animal reservoirs, such as influenza and coronaviruses, can have a detrimental impact on society. Vaccines that are available against influenza, SARS-CoV-2, and RSV can mitigate the substantial burden arising from these infections, and new combination vaccines are in development by several manufacturers with the aim to increase their effectiveness and facilitate vaccination practices.
In the following sections, we take a closer look at these topics through expert presentations from ESCMID Global 2024.
Current Epidemiology of Respiratory Viruses and Diagnostic Approaches
During the COVID-19 pandemic, containment measures led to a reduction in the transmission of respiratory viruses, such as influenza and RSV.3 After the COVID-19 pandemic, the WHO established an integrated surveillance to monitor respiratory viruses of public health importance against which an intervention is available, focusing on influenza, SARS-CoV-2, and RSV,4 particularly as there is co-circulation of these viruses along with other respiratory pathogens.5
During the symposium session titled ‘Aiming at a moving target with the best flu shot’, Ben Cowling, Professor of Epidemiology at the University of Hong Kong, Hong Kong, noted changes in the seasonality of respiratory viruses, with variations in the timing of influenza peaks globally, and the disappearance of the influenza B Yamagata lineage.6 With no detection of the B Yamagata virus since before the pandemic, the WHO has urged the manufacturers to remove this component from annual influenza vaccines.6 Cowling highlighted the need to take extra caution in laboratories working with this virus to prevent spillover back into circulation. Cowling also cautioned vaccine developers to consider the circulation patterns of different viruses to develop next-generation combination vaccines, to provide protection, at least throughout the season, for each virus included in the vaccine.
Following the COVID-19 pandemic, there has also been an increase in viral testing and assay development.7,8 Diagnostics are crucial for patient care and control measures to limit transmission, such as detecting emerging viruses, determining vaccine efficacy, and assisting in treatment decision-making, thus informing public health measures.4 During the ’Seasonal influenza, COVID, and RSV: where do we go?’ session, Sylvie van Der Werf, Professor at the University Paris Diderot in France, highlighted the importance of laboratory-based and point-of-care diagnosis for detecting multiple viruses, bacteria, and variants of concern.
The current gold-standard laboratory-based diagnostic method for respiratory viruses is primarily nucleic acid amplification assays, particularly real-time quantitative polymerase chain reaction.9 Such methods offer high clinical and analytic performance, with low error and intra- and inter-assay variability, thereby reducing the length of hospital stays through improved and timely treatment approaches.10-13 However, these tests require trained personnel, are sensitive to mutations, and are dependent on specimen quality, which is crucial for treatment decision-making.11,13 van Der Werf also highlighted the need for respiratory virus diagnosis to focus on point-of-care multiplex platforms, including tests for emerging variants, predictive biomarkers, and progression to severity, while ensuring equitable global access to tests for pandemic preparedness. Both Cowling and van der Werf emphasised the need for an integrated surveillance system that utilises robust diagnostic tools for accurately assessing circulating viruses, detecting emerging strains, and informing future vaccine development strategies.
How Much Sequencing is Enough to be Able to Detect Viruses of Pandemic Potential and Emerging Variants During a Pandemic?
In addition to routine surveillance based on diagnostic tests, genomic surveillance, which involves routine sampling from an individual who tests positive for a virus, and is subsequently sequenced for the pathogen’s genome,7 is the cornerstone of public and global health. This allows for the timely detection of potentially highly transmissible viral variants before or during a pandemic.
During the ‘Seasonal influenza, COVID, and RSV: where do we go?’ symposium, Colin Russell, Professor of Infectious Diseases from the University of Amsterdam UMC, the Netherlands, highlighted the importance of global solidarity and mutual interdependence in monitoring viral evolution, transmission, and detection of emerging viruses. Using a global metapopulation epidemic modelling approach, the time-to-detection was strongly associated with where variants first emerged, and the sequencing capacity of a particular country. Russell pointed out the heterogeneous capacity for sequencing virus genomes worldwide, with some countries sequencing significantly more genomes per million people per week than others during the COVID-19 pandemic.8 The model found that virus sequencing above 10 sequences per million people per week had limited additional capacity regarding detection time.8 However, a modest increase in surveillance, in countries where very limited sequencing (<2 virus sequences per million people per week) was occurring, led to considerable benefits in time-to-variant detection capacity.8 This inequitable distribution of genomic surveillance capacity had a detrimental impact on the global capacity to efficiently detect new viral variants. Russell proposed establishing a minimum global sequencing capacity of two sequences per million people per week, to effectively monitor variant prevalence for respiratory viruses like influenza and SARS-CoV-2. This highlights the importance of improving international solidarity in global respiratory virus genomic surveillance to reduce the time needed to detect new variants and to safeguard public health.
Influenza: An Infection That Can Trigger Heart Attacks, Bacterial Pneumonia, and Can Lead to Excess Use of Antibiotics
Data since the early 1900s indicate that influenza might increase the risk for cardiovascular complications. Today, we know that influenza can go beyond simple respiratory infection, potentially triggering heart attack, stroke, and bacterial pneumonia, and can contribute to antimicrobial resistance (AMR).14,15
Mine Durusu Tanriover, Professor of Internal Medicine at Hacettepe University, Türkiye, highlighted in the session ‘Aiming at a moving target with the best flu shot’ that there is a need to understand the true burden of influenza, and the associated increased risk of pneumonia, cardiovascular, and cerebrovascular complications. For instance, an observational study from Denmark from 2010–2016 reported that, within 3 days of laboratory-confirmed influenza infection (LCI), the risk of having a heart attack and stroke increased by 17.5 (95% CI: 8.5–36.2) and 10.3 (95% CI: 4.2–25.4) times, respectively.16 A recent (2017–2020) prospective multi-centre surveillance study from Spain identified that 5.5% of patients (43 patients) admitted to ICUs with acute cardiac complications were diagnosed with LCI, with 37.2% of these showing no symptoms of influenza infection.17 These influenza-positive patients had higher rates of heart failure and required more medical support.17
In accordance with Durusu Tanriover’s comments, a poster presented by Muñoz-Quiles, a researcher at FISABIO, Valencia, Spain, explored the association between clinically diagnosed influenza (CDI) in a primary care setting and LCI in a hospital setting, and acute cardiovascular events (ACVE), heart attack, and ischaemic stroke.14 Data were collected from electronic healthcare registries using a population-based, retrospective, self-controlled case series design, where individuals act as their own control, avoiding biases in individuals’ differences in baseline time-invariant characteristics. All patients were aged ≥50 years, with at least one ACVE and one influenza episode (n=2,230,015).14
The study found the risk of ACVEs was significantly higher after both CDI and LCI, with LCI (n=625) patients showing a higher mortality rate (43%) compared with overall (14%) and CDI (18%) populations.14 The incidence of ACVEs was significantly higher during the 14-day period after CDI (incidence rate ratios [IRR]: 2.21 and 2.62 during 1–7 and 8–14 days, respectively), and during the 60-day risk period after LCI (4.40, 5.09, 2.47, and 2.24 during 1–7, 8–14, 15–29, and 30–60 days, respectively), compared with baseline.14 The risk of ACVEs more than doubled (IRR: 2.62; 95% CI: 1.51–4.52) in patients aged ≥50 years with CDI at 14 days, and quadrupled in patients aged ≥65 years (IRR: 5.66; 95% CI: 2.79–11.43) with LCI, remaining elevated for 60 days (IRR: 2.48; 95% CI: 1.43–4.29).14 These data supported the official recommendations for influenza prevention in at-risk groups and possible complications following CDI.
Influenza Vaccines Help Reduce the Risk of Cardiovascular Complications and Use of Antibiotics
During her talk, Durusu Tanriover also emphasised the importance of vaccination in the prevention of cardiac complications, citing a meta-analysis of six RCTs (n=9,340), which demonstrated that influenza vaccination was associated with a reduced incidence of the primary composite endpoint (cardiovascular death, acute coronary syndrome, stent thrombosis, coronary vascularisation, and hospitalisation due to stroke or heart failure in patients with existing heart disease) by 26% (random effects hazard ratio [rHR]: 0.74; 95% CI: 0.63–0.88; p<0.001), cardiovascular death by 37% (rHR: 0.63; 95% CI: 0.42–0.95; p=0.028), and all-cause death by 28% (rHR: 0.72; 95% CI: 0.54–0.95; p=0.0227).15
According to data presented by Durusu Tanriover, vaccination may reduce inappropriate antibiotic prescribing by reducing the incidence of seasonal influenza complicated cases.18 A systematic literature review of 17 RCTs found a 29% reduction in the proportion of people receiving antibiotics after influenza vaccination (risk ratio: 0.71; 95% CI: 0.62–0.83) and a 37% reduction in the number of antibiotic prescriptions, or days of antibiotic use after influenza vaccination (risk ratio: 0.63; 95% CI: 0.51–0.79).18
Durusu Tanriover concluded that preventing respiratory infections through vaccination can decrease the risk of clinically significant morbidity and antimicrobial use, thereby supporting antibiotic stewardship. She concluded her presentation by emphasising the importance of properly utilising the vaccines to reduce the burden of influenza.
Need For High-Quality Evidence: Randomised Trials/Studies
Study methodologies that are utilised to estimate vaccine performance are very important as these estimations are used for decision-making and vaccine recommendations. Therefore, robust methodologies such as RCTs are essential to establish a causal relationship between the intervention and the outcome. However, RCTs can be costly and cumbersome to perform on a large scale. During the symposium session ‘Which randomised controlled trial do we need?’, Guy Thwaites, Professor of Infectious Diseases at the Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam, highlighted the importance of large, simple, pragmatic RCTs in the infectious disease field, despite the challenges of large sample sizes and modest effects. However, he noted that few such pragmatic RCTs have been conducted, and noted challenges in trial design including endpoints, sample size, funding constraints, and early termination issues.
The 2020 RECOVERY trial, which assessed COVID-19 treatments, marked a new era of large adaptive pragmatic platform trials (enrolling n=49,204 participants across 184 sites at the time of this presentation).19 However, Thwaites acknowledged that conducting pragmatic RCTs in countries without national healthcare systems is challenging, leading to the emergence of trial consortiums and networks. Thwaites believes that the increased bureaucracy and clinical trial costs impede the advancement of much-needed RCTs for urgent treatment needs.
Thwaites acknowledged a need for sustainable clinical trial platforms and networks, in addition to large, pragmatic clinical trials that address real-world clinical practice questions that physicians encounter every day. Thwaites called for more investment in the regulatory systems, specifically in low- and middle-income countries, and innovative trial designs to deliver trials in a collaborative and co-coordinated manner to progress science.
Developing Vaccine Recommendations Through Comprehensive Evidence Synthesis: Grading of Recommendations, Assessment, Development, and Evaluation Analysis as an Example
During the symposium session ’Aiming at a moving target with the best flu shot’, Nigar Sekercioglu, Associate Professor of Medicine at McMaster University in Hamilton, Canada, considered the importance of evidence synthesis and methodology to formulate research questions, particularly for systematic reviews and guideline development.20,21 Sekercioglu stated that developing a well-defined question for systematic reviews, and supporting the development of evidence for decision frameworks, is very important, and should clearly define the patient population, interventions, comparators (or comparisons), and outcomes (PICO framework).20,21
Systematic reviews of RCTs are a key evidence base for creating guidelines in clinical practice, public health, and health policy (including coverage decisions) and recommendations.22-27 The use of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology for formulating recommendations in the context of systematic reviews outlines the differences in question development and requires clear PICO specification.28
When evaluating the quality of evidence, the five domains of the GRADE approach can be used for both RCTs and observational studies (Table 1), whilst also considering any other factors that may influence the certainty of the evidence.29
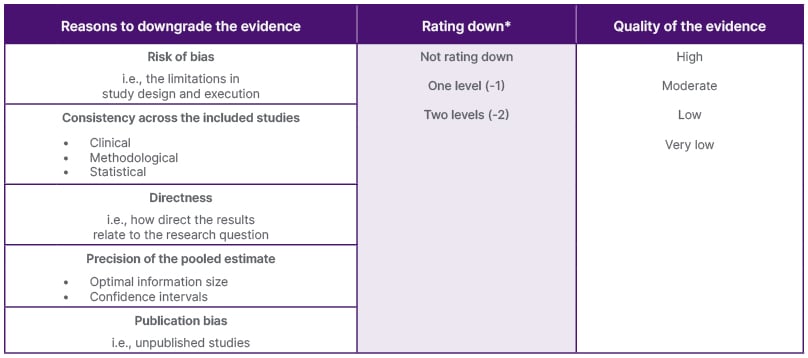
Table 1: Assessment of certainty of evidence within the context of pair-wise.29
*May go as low as three levels (-3) in the precision domain, e.g., in network meta-analysis.
Sekercioglu also noted that guidelines should be developed by knowledgeable multi-disciplinary expert panels, including representatives from key stakeholders, and should use the GRADE ‘evidence to recommendations’ framework.30 The framework involves balancing desirable and undesirable intervention consequences, including efficacy and safety, certainty of the evidence of effects, patient values and preferences, resource requirements, cost-effectiveness, equity, acceptability, and feasibility for all key stakeholders.31 This is to ensure guidelines are of high-quality, trustworthy and implementable, and follow an explicit and transparent process, as outlined by the GRADE working group.29
The GRADE system’s advantages include applicability, transparency, clear rules for adjusting the quality of evidence, and explicit acknowledgement of values and preferences.30 Separate decisions for the quality of evidence and strength of recommendations can be made strong or conditional, and directional (for, or against).30 For example, strong indicates a ‘recommend’, weak or conditional indicates ‘suggest’, and no grade indicates ‘it is reasonable to do’.30
In the GRADE methodology, the evidence hierarchy (Figure 1), consists of critical appraisal in the form of well-designed systematic reviews and meta-analyses at the top. This is followed by experimental studies such as RCTs, which Sekercioglu stated as “the highest quality of evidence for interventional questions”, and considered the gold standard. However, no study design is uniformly perfect. Observational studies such as cohort studies, case studies, and expert opinion are at the bottom of the hierarchy, which may be subject to causal inferences depending on fulfilling the Bradford Hill criteria of causation.32
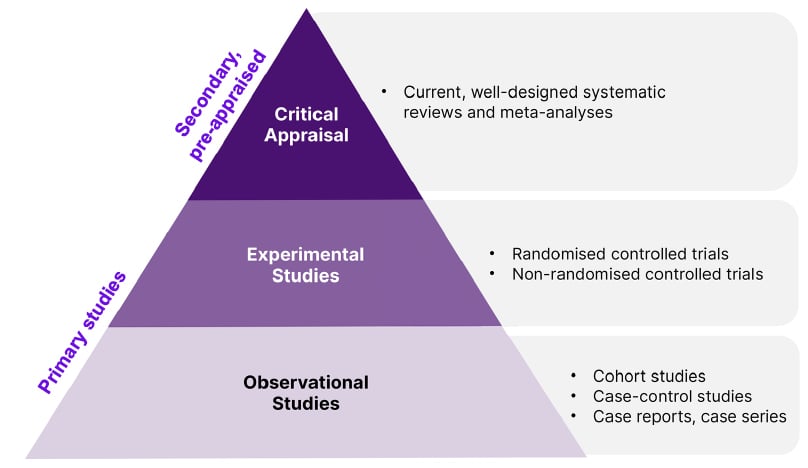
Figure 1: Hierarchy of evidence pyramid.
Sekercioglu stated that the correct choice should be based on the research question, available resources, and context. The main source of input for guidelines is systematic reviews, as they provide evidence on both the desirable and undesirable consequences, as well as for other domains of the framework. Judgements for all the relevant domains should be made to facilitate systematic consideration of contextual criteria relevant to each guideline question, which can be developed in a structured and transparent manner.
Future Approaches to Efficient Clinical Trial Design
Looking at how to innovate the clinical trial space and ensure efficiency, Lauren Maxwell, Senior Epidemiologist and Data Engineer at the Heidelberg Institute of Global Health, Germany, emphasised the value of data-driven medical applications during the ‘Which randomised controlled trial do we need?’ symposium session.
Maxwell indicated that semantic and syntactic interoperability, preserving data meaning and relationships when reusing data, is the most difficult to address in clinical trials.33 FAIR-by-design is the prospective implementation principle (Table 2), where data should be findable, accessible, interoperable, and reusable (FAIR), focusing on metadata, and understanding data reusability.33
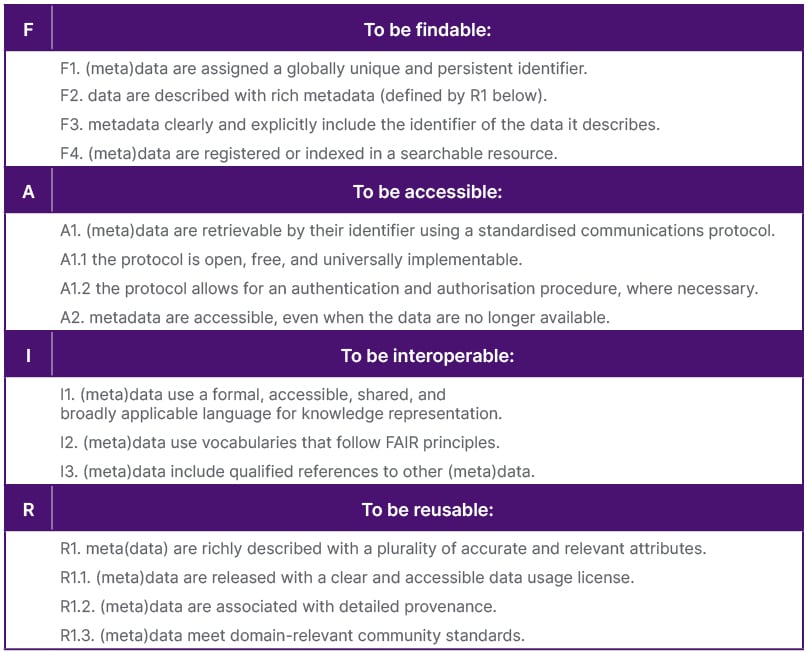
Table 2: The FAIR Guiding Principles.33
FAIR-by-design aids in understanding the real-world trial landscape and retrospective analysis by combining different data sources.34 This, Maxwell noted, requires an understanding of site-level variability, proficiency of site onboarding, recruitment and retention factors, and trial overlap to connect participant-level data from multiple data sources without data coarsening. Despite the advantages, FAIR data adoption is limited due to a lack of capacity and funding. However, Maxwell highlighted the importance of considering clinical trial data and reusing data as a valuable asset.35
Another approach, ‘federated learning’, reuses data without moving it. Machine-learned models are trained over multiple databases without sharing raw data by following a privacy-by-design approach.36 This is useful for identifying trial participants, improving site selection, and including laboratory data and data across multiple trials.36 Maxwell identified that federated data is beneficial when data is not open, transferable, or needs to be accessed quickly. However, it should not be used for metadata, low-quality data, or when permissions are not interoperable or granted. Fundamentally, federated data reuse depends on FAIR principles and data/metadata quality.
Maxwell also emphasised the potential of AI in the clinical trial landscape, for study recruitment, participant identification, and the use of digital twins (the identification of individuals at a particular disease state to reduce the required size of the control arm), where AI can streamline protocols, save time, link up study documentation, and explain non-linearity.37,38
Additionally, decentralised trials, which are conducted remotely, use toolkits such as eConsent, devices or sensor data, telemedicine, and logistic support, such as shipping products or diagnostics to participants.39 These trials improve patient safety through continuous monitoring and a more reliable assessment of treatment efficacy or trial endpoints.40 Maxwell highlighted that decentralised trials have the potential to increase and diversify recruitment, enabling equity and inclusion in trials. Maxwell also noted, however, that these trial approaches lack national policies, and may exclude non-tech-savvy individuals, or overburden participants with unnecessary technology or complexity. Despite these issues, they are similar to those seen with a traditional RCT design, and Maxwell indicated that this approach could impact infection prevention and safety monitoring, reduce cost, and increase efficiency.
Across AI, decentralised, and federated learning, high-quality FAIR data and metadata are crucial. Investing in data is as important as AI innovation. The challenge of aligning data with existing ontologies and standards highlights that, even with efforts to ensure FAIR data, compliance with standards may not be achieved. Maxwell highlighted the importance of building a community around data reuse in infectious diseases, which can help regulators understand effective approaches.
Conclusion
Influenza and other respiratory viruses are back in circulation after the COVID-19 pandemic, with one influenza B virus, B/Yamagata, not being reported since the beginning of the pandemic, which led the WHO to recommend switching back to trivalent formulation of influenza vaccines. Influenza may lead to heart attacks, stroke, and, furthermore, bacterial pneumonia and excess use of antibiotics, which can in turn contribute to AMR. Currently, there are enhanced vaccines that are recommended for the older populations, and there might be more vaccines available in the future. Therefore, the generation of high-quality evidence on the performance of these vaccines is needed for informed decision-making when recommending the best possible vaccine to the most vulnerable populations. Currently, RCTs are the gold standard in assessing the performance of medicinal products; however, due to cost and difficulties associated with RCTs, new approaches are also explored by scientists to generate robust data. In addition to generating robust data, robust methodologies are also needed to synthesise the available data to formulate recommendations. Currently, the GRADE methodology, which also ranks RCTs as the gold standard in generating robust data, is used by several health authorities.
It is clear that, while timely and accurate diagnosis of respiratory viruses have an impact on the clinical prognosis of patients, well-established viral and genomic surveillance are essential for the assessment of epidemic burden at a public level, and for early detection of new virus variants of pandemic potential. In summary, continued research, collaboration, and evidence-based interventions for the advancement in vaccine development and testing are essential to address the burden of these respiratory viruses, their long-term effects, and their mitigation.







