Abstract
Background: Antimicrobial resistance (AMR) is a critical global issue, contributing to increased morbidity and mortality, prolonged hospital stays, and rising healthcare costs. In Afghanistan, alarming levels of AMR continue to be driven by self-medication, overuse of antibiotics, and inadequate awareness among patients and healthcare providers. This study aimed to evaluate the levels of AMR at the French Medical Institute for Mothers and Children (FMIC) hospital in Kabul, Afghanistan, and compare them with the global landscape of AMR.
Methods: Blood, urine, and purulent samples were collected from 6,709 patients at FMIC hospital, including infants, children, and adults. The disk diffusion method was used for antimicrobial susceptibility testing, and data analysis was performed using SPSS (IBM, Armonk, New York, USA) software.
Results: The majority of participants were adults (45.8%) and female. The largest proportion of positive samples came from male patients, and urine cultures yielded the highest number of positive results. Escherichia coli was the most commonly isolated microorganism, with resistance found in 51.2% of patients, followed by Staphylococcus aureus with resistance levels of 26.37%. Amikacin was the most frequently used antibiotic, while amoxicillin/clavulanic acid was the least effective. Levels of E. coli resistance were seven-to-eight times higher than in Europe, highlighting the urgent need for improved antibiotic stewardship and infection control measures in Afghanistan.
Conclusion: The findings provide valuable information on the prevalence of microorganisms in clinical samples and their susceptibility to various antibiotics in Kabul, contributing to the development of effective strategies to combat AMR both locally and globally.
Key Points
1. Antimicrobial resistance (AMR) is a growing global health threat, exacerbated by overuse and irrational use of antibiotics, particularly in low-resource settings like Afghanistan, where testing centres are limited.
2. This study, conducted at FMIC hospital in Kabul, analysed microbial resistance patterns among 6,709 patients, highlighting alarmingly high antibiotic resistance rates to common antibiotics like ampicillin and ciprofloxacin.
3. The study underscores the need for strict antibiotic stewardship and rational prescription practices, particularly for clinicians who overuse antibiotics, to combat the rising threat of AMR in Afghanistan and similar regions.
INTRODUCTION
Antimicrobial resistance (AMR) is a critical global issue, contributing to increased morbidity and mortality, prolonged hospital stays, and rising healthcare costs. According to the WHO, microbial resistance is expected to be one of the leading causes of death by 2050, surpassing even cancer. Currently, around 700,000 people die annually due to infections resistant to common antibiotics.1,2
Existing studies reveal alarming levels of AMR in Afghanistan, driven by factors such as self-medication, overuse of antibiotics, and inadequate awareness among patients and healthcare providers. For instance, findings show that 87% of individuals procure antibiotics from pharmacies without prescriptions, and misconceptions about antibiotic usage are prevalent.3 Additionally, local studies have identified high resistance rates among both Gram-positive and Gram-negative bacteria, particularly for commonly used antibiotics such as ampicillin and ciprofloxacin. Despite these challenges, limited data exists comparing microbial resistance in Afghanistan to global trends. This study addresses this gap by evaluating resistance patterns in Kabul hospitals and comparing them with other countries. This contribution is critical to informing targeted interventions for reducing AMR in the region and aligning Afghanistan’s response with global efforts.
In Afghanistan, AMR is exacerbated by various factors, including limited access to healthcare, low literacy, and the availability of substandard antibiotics. A 2021 study found that many people in Afghanistan use antibiotics irrationally, either without prescriptions or in combination with other drugs, in an attempt to accelerate recovery.4 Such practices increase the risk of resistance, which can worsen the clinical outcomes of infections and complicate treatments. In a 2021 study in Kabul5 and a study conducted at FMIC between 2010–2015, resistance rates to commonly used antibiotics, such as amoxicillin and ampicillin, were alarmingly high.6
This study is of great significance for Afghanistan as it provides crucial insights into the rising levels of AMR in the country, a growing public health threat. Understanding local resistance patterns is essential for developing targeted treatment strategies, improving antibiotic stewardship, and guiding public health policies to combat infections effectively.
Globally, the study contributes to the broader understanding of AMR by highlighting regional differences in resistance profiles and comparing Afghanistan’s data with international trends. It emphasises the need for global collaboration in addressing AMR, sharing knowledge, and implementing effective measures to prevent its spread. The findings also add to the growing body of research on antibiotic resistance, which is critical for informing worldwide strategies to preserve the effectiveness of antibiotics for future generations.
MATERIALS AND METHODS
This cross-sectional descriptive study was conducted from January–November 2023 at FMIC hospital, a leading hospital in Kabul. A total of 6,709 patients were screened for microbial cultures and antibiograms, with 1,889 positive cases analysed after excluding irrelevant samples (e.g., fungal samples and contaminated samples). The patient data collected included demographic details, the nature of the sample (e.g., blood, urine, or purulent), and the specific antibiotics tested.
Samples were processed in the laboratory using standard diagnostic procedures to ensure reliability and accuracy. Blood cultures were analysed using the BACTEC™ 9240 machine, and bacterial identification was performed using API identification strips (Biomerieux, France). Antibiotic susceptibility testing was conducted using the disk diffusion method, strictly adhering to Clinical and Laboratory Standards Institute (CLSI) guidelines.7 Internal quality control measures included the use of control strains and regular calibration of laboratory equipment. Additionally, any unexpected or inconsistent results were repeated to confirm accuracy. The laboratory staff consisted of trained microbiologists. Supervisors regularly reviewed the work to ensure strict adherence to established protocols.
Data were analysed using SPSS version 25 and Microsoft Excel (Redmond, Washington, USA), with a focus on patient demographics, types of microorganisms identified, and antibiotic resistance patterns. Patient information was obtained from the healthcare system database, and there was no direct contact with patients.
This study was conducted in coordination with the Ministry of Public Health of Afghanistan and the management of the hospital. Ethical approval was granted by the Ethical Committee of Rabia Balkhi University, Kabul, Afghanistan, ensuring compliance with national and international ethical standards for research.
RESULTS
This study, conducted in 2023, aimed to assess the microbial resistance situation in Kabul city. A total of 6,709 samples were collected, of which 1,889 exhibited positive bacterial growth and were subsequently analysed. The samples were categorised into three age groups: infants (from birth to 2 years old), comprising 831 samples (44%); children (ages 2–19), comprising 192 samples (10.2%); and adults (ages 19–100), comprising 866 samples (45.8%). Among the participants, 910 (48.1%) were men and 979 (51.8%) were women. Regarding the sample origin, 318 blood samples (16.8%), 799 urine samples (42.3%), and 772 pus samples (40.9%) were analysed.
In this study, most infants were male. The frequency of participation among infants and children was similar across genders, while female adult participants were significantly more common (55.2%). Most blood samples were obtained from infants (82.4%), while most urine and purulent samples were collected from adults. The study identified 27 different microorganisms, with the most common resistant organisms being Escherichia coli (27.7%), predominantly in female patients (66.09%), followed by Staphylococcus aureus (13.8%), Staphylococcus spp. (non-aureus; 13.5%), Pseudomonas spp. (8.5%), Serratia spp. (4.9%), Klebsiella spp. (4.6%), Enterococcus spp. (3.4%), and Serratia odorifera (2.8%), with 55.77% of cases occurring in females.
ANTIBIOTIC RESISTANCE PATTERNS
Among the 33 antibiotics tested, the following showed the highest resistance rates:
- Amoxicillin-clavulanic acid: 81.9% resistance with 51.25% in females.
- Ampicillin: 95.7% resistance with 52.22% in females.
- Cefixime: 86% resistance with 51.85% in females.
- Cotrimoxazole: 68% resistance with 53.07% in females.
- Ceftazidime: 62.4% resistance with 52.21% in females.
- Ofloxacin/ciprofloxacin: 56.6% resistance with 52.72% in females.
Most significantly, higher resistance was observed in females, but antibiotics such as amikacin (56.42%), piperacillin/tazobactam (57.14%), meropenem (64.57%), rifampicin (56.12%), vancomycin (66.66%), chloramphenicol (70.27%), tetracycline (58.33%), and fosfomycin (57.89%) showed higher resistance in male participants than in females (Table 1).
Antibiotics with the least resistance (below 20%) included vancomycin (2.4% resistance), colistin (2.6% resistance), and polymyxin B (0% resistance) (Table 2).
Resistance to antibiotics such as ampicillin and amoxicillin-clavulanic acid was notably high, indicating widespread resistance among common pathogens.
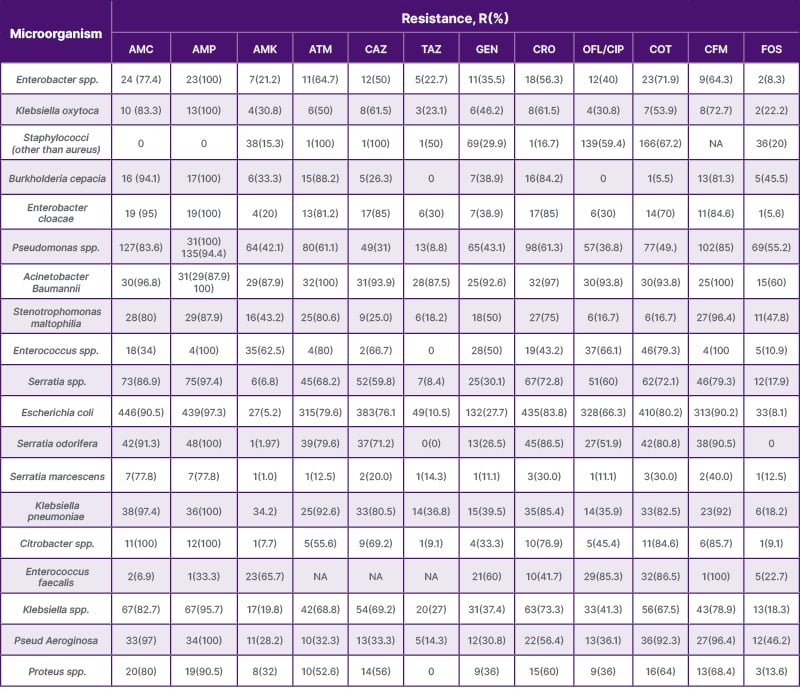
Table 1: The most common antibiotic-resistant microorganisms identified in this study.
AMC: amoxicillin + clavulanic acid; AMK: amikacin; AMP: ampicillin; ATM: aztreonam; CAZ: ceftazidime; CFM: cefixime; COT: co-trimoxazole; CRO: ceftriaxone; FOS: fosfomycin; GEN: gentamicin; NA: not applicable; OFL/CLP: ofloxacin/ciprofloxacin; TAZ: piperacillin + tazobactam.
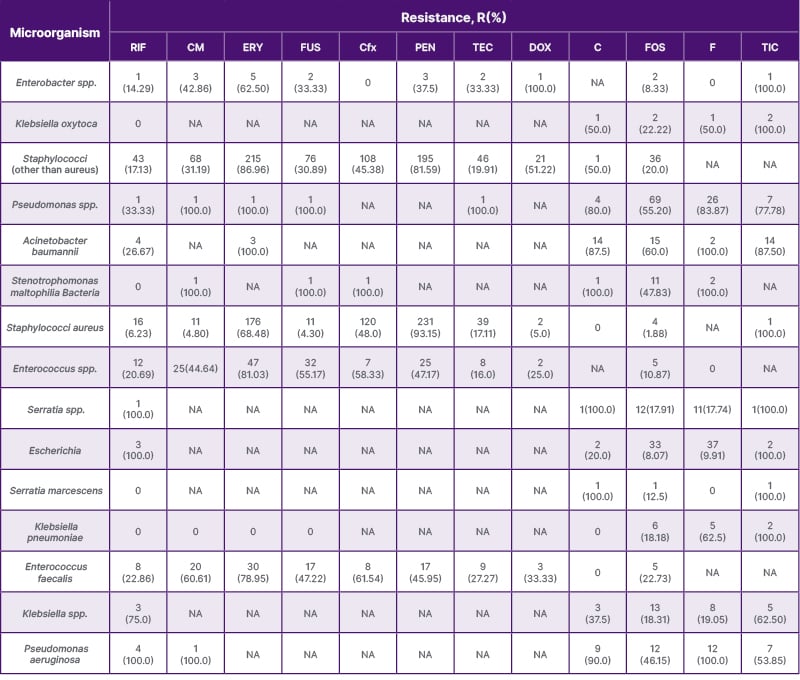
Table 2: The antibiotic-resistant microorganisms least frequently used in this study.
C: chloramphenicol; CFx: cefoxitin; CM: clindamycin; DOX: doxycycline; ERY: erythromycin; F: nitrofurantoin; FOS: fosfomycin; FUS: fusidic acid; PEN: penicillin; RIF: rifampicin; TEC: teicoplanin; TIC: ticarcillin.
DISCUSSION
The findings of this study align with global trends, showing a significant increase in AMR. Escherichia coli was found to have the highest resistance to cephalosporins, penicillins, and fluoroquinolones, which mirrors findings from other regions, including Iran and Morocco.8,9 Additionally, resistance to co-tromoxazole has been reported in Iran.10
When comparing these results with other countries, such as Iran, China, and the USA,11 Afghanistan’s resistance rates were found to be significantly higher. For example, E. coli resistance to cephalosporins in Europe averages around 12%, whereas in Afghanistan, it was found to be 80.23%.12 A study in a US military hospital found that 70% of E. coli strains among Afghanistan nationals are resistant to cephalosporins.13 This substantial difference highlights the urgent need for improved antibiotic stewardship. Infection control practices in Afghanistan also require significant attention.
Among Gram-positive organisms, Staphylococcus aureus demonstrated 93.14% resistance to penicillin, which is greater than the 99.05% observed in this study, underscoring the prevalence of methicillin-resistant S. aureus (MRSA) in the region.14 However, resistance to vancomycin was very low, which is promising for the treatment of MRSA infections in Afghanistan. In Kathmandu, Nepal, S. Aureus is less resistant to erythromycin, co-trimoxazole, and ciprofloxacin than in Afghanistan.15 In a 2008 study conducted in the European Union (EU), Staphylococcus spp. exhibited an overall resistance rate of 34.78% to fusidic acid.16 Among European countries, Ireland showed the highest resistance at 50%, followed by France with 49.4% resistance.16 In the authors’ study, Staphylococcus spp. (coagulase-negative) in Afghanistan showed a low resistance rate. This difference may be due to the lower consumption of fusidic acid in Afghanistan.
In a study conducted in 2007–2008 in the USA, only 7.2% of Staphylococcus spp. exhibited resistance to fusidic acid; while in Canada, the resistance rate was 20%, and in Australia, it was 10.8%.16 All of these rates are considerably lower than the resistance rate observed in Afghanistan.
Pseudomonas spp., another common pathogen, exhibited high resistance to multiple antibiotics, including amoxicillin-clavulanic acid (83.55%) and ampicillin (94.41%). This is concerning, as Pseudomonas infections are often associated with severe, hospital-acquired infections that require potent antibiotics. The authors’ study shows a concerning increase compared to previous studies. For instance, in Kandahar, Afghanistan in 2022, the resistance rate of Pseudomonas spp. to nitrofurantoin was recorded at 21.7%; whereas, this study found a much higher resistance rate of 83.87%.17 This sharp rise suggests a significant increase in resistance over a short period, highlighting the potential challenges in treating infections with nitrofurantoin. Similarly, amikacin and ceftriaxone resistance in Pseudomonas spp. was found to have increased between 2022–2023.17 This increase suggests a growing resistance to antibiotics, which is often used as a second-line or even last-resort antibiotic for treating resistant Pseudomonas infections.
According to the authors’ research, the overall antibiotic resistance of Serratia spp. in Afghanistan is 51.19%. In a 2022 study conducted in Kandahar, Serratia spp. exhibited a resistance rate of 53.8% to amoxicillin and ampicillin, 25.7% to cephalosporins, 15.4% to imipenem, 15.4% to fluoroquinolones, and 38.5% to vancomycin.17 In comparison, this study found a significantly higher rate of resistance; in some cases, two times more than in Kandahar, Afghanistan.
In a 2018 study conducted in Iran, Klebsiella spp. showed 54% resistance to co-trimoxazole.10 In Mexico (2005–2010), Klebsiella spp. showed an overall resistance rate of 23.36% to cephalosporins, 17% to fluoroquinolones, and 11.2% to piperacillin/tazobactam.18
In a 2016 study in eastern India, Streptococcus pyogenes showed 0% resistance to penicillin G, cefotaxime, vancomycin, and clindamycin, while erythromycin had a resistance rate of 2.85%, and tetracycline resistance was 53.57%.19 In contrast, in the authors’ study, Streptococcus pyogenes showed equal or high rate of resistance.
Regarding Streptococcus pneumonia, in a study by Jae-hoon Song et al.20 in Korea (2000–2001), S. pneumonia strains were less resistant to antibiotics than in Afghanistan, including penicillin, amoxicillin/clavulanic acid, erythromycin, and ceftriaxone. Furthermore, Song et al. reported that in other countries, S. pneumoniae had an overall resistance rate of 29.4% to penicillin, 32.4% to ceftriaxone, 53.1% to erythromycin, 1.6% to levofloxacin, and 6% to ciprofloxacin. When comparing the authors’ results to data from other countries, they found that the resistance to penicillin and erythromycin in Kabul is approximately twice as high.
The significant differences in AMR between Afghanistan and other countries can be attributed to several factors, including the overuse, over-prescription, and misuse of antibiotics, as well as the availability of non-standard or counterfeit antibiotics. Other contributing factors include poor antibiotic stewardship, inadequate infection control measures, limited access to healthcare, diagnostic challenges, ineffective waste management practices, a low-income economy, and low health literacy. In Afghanistan, antibiotics are often used without proper prescriptions, leading to misuse and an increase in resistance. This is less common in countries with stricter regulations. Usman Hadi and their colleague’s studies in Indonesia have highlighted the necessary actions for reducing this, including modifying the behaviour of healthcare professionals and hospital staff, adapting health preservation practices, and enhancing good laboratory methods to decrease microbial resistance.21
Differences in laboratory materials, methods, and training could be significant factors in the observed discrepancies in antibiotic resistance results between Afghanistan and other countries. Standardising testing procedures and improving laboratory quality would help reduce these discrepancies.
CONCLUSION
This study highlights the alarming rates of AMR at this hospital in Kabul, Afghanistan, reflecting broader trends observed in similar low-resource settings. The findings emphasise the urgent need for action to address the growing AMR crisis in Afghanistan. Addressing this issue in a country with limited healthcare infrastructure presents significant challenges. Key strategies should include strengthening infection control measures, improving antibiotic stewardship, regulating the quality and availability of antibiotics, and promoting public awareness about the dangers of self-medication and overuse of antibiotics. Additionally, future research should explore new therapeutic options, including the use of biotechnology and nanotechnology solutions, to combat drug-resistant pathogens. This will require investment in local research capacity, partnerships with international experts, and the development of affordable treatment alternatives for the population in Afghanistan.
Given the literacy challenges, effective education for both healthcare workers and the public is critical. This can be achieved through the use of visual aids, community outreach, and culturally relevant messaging that emphasise the risks of improper antibiotic use. Healthcare workers can play a central role by leading these educational initiatives, ensuring that messages are delivered in simple, accessible ways.
In light of the high levels of resistance observed in this study, it is clear that AMR is an escalating problem in Afghanistan. Immediate action is needed from healthcare authorities, policymakers, and the international community to implement strategies that protect public health and prevent further resistance.







