Meeting Summary
Transradial access (TRA) is a key strategy to avoid bleeding during percutaneous coronary intervention (PCI) and coronary angiography, as it reduces the risk of bleeding associated with transfemoral access (TFA). The distal radial artery (dRA) is located at the anatomical snuffbox, or radial fossa, which is a triangular depression on the lateral aspect of the dorsum of the hand. Distal transradial access (dTRA) at the anatomical snuffbox has recently gained interest as a non-femoral alternative access route for vascular procedures. Opportunities and challenges in the TRA/dTRA space include adoption of different and more complex procedures, technical innovation in access and haemostasis, and financial savings for institutions and healthcare systems. Historically, the adoption of TRA has been concentrated among interventional cardiologists, with much less adoption in the interventional radiology, vascular surgery, and neurointerventional radiology communities. This article highlights key aspects of the Think Radial webinar, an educational training session conducted by Merit Medical. The training session was conducted by three experts: Sandeep Nathan, Department of Cardiology, University of Chicago Medicine, Illinois, USA; Darren Klass, Department of Radiology, University of British Columbia, Vancouver, and Department of Radiology, Vancouver General Hospital, British Columbia, Canada; and Ajit Puri, New England Center for Stroke Research, Department of Radiology, University of Massachusetts Medical Center, Worcester, USA, all of whom have a wealth of experience in vascular access. The experts provided valuable insights into topics such as why and how to adopt a dTRA approach, the value of ultrasound for guided vascular access, and dTRA and ultrasound tips and techniques. The experts also explored the challenges in transradial intervention and management of radial access complications. Drawing on their individual specialist knowledge, the experts concluded with presentations on PCI, radial approaches in interventional radiology, and dRA access in neurointervention. The aim of this educational article is to disseminate key information on TRA provided by the experts to guide and update healthcare practitioners, particularly interventional cardiologists and interventional radiologists, working in vascular access.
INTRODUCTION
The radial artery (RA) descends along the lateral side of the forearm above the radius towards the wrist. The dRA is located at the anatomical snuffbox, or radial fossa, which is a triangular depression on the lateral aspect of the dorsum of the hand. TRA for diagnostic angiography was first described in 1989 by Lucien Campeau1 as a new way to generate diagnostic images. Four years later, Ferdinand Kiemeneij introduced the TRA approach for coronary intervention.2 Considerable evidence supports transradial angiography and intervention in patients with acute coronary syndrome, with an emphasis on decreasing major bleeding and access site vascular complications.3 dTRA at the anatomical snuffbox has recently gained interest as a non-femoral alternative access route for vascular procedures.
WHY SHOULD I CARE ABOUT A DISTAL TRANSRADIAL APPROACH?
Reasons for Adopting a Distal Transradial Approach
Healthcare professionals who utilise conventional radial techniques may question the importance of a dTRA approach. However, there are several reasons why they should care. Most notably, TRA is associated with reduced rates of bleeding, cardiovascular complications, and mortality compared with conventional access.4-6 In addition, dTRA is a safe, non-femoral access point for the majority of catheter-based interventions. This technique optimises operator comfort and ergonomics, particularly for left-dTRA in individuals with obesity, or if conducting radial to peripheral procedures, and improves patient comfort (e.g., the hand is pronated rather than supinated). Furthermore, dTRA is a safe approach for recanalising an occluded proximal RA, and may provide more rapid radial haemostasis than conventional radial access, while also potentially being associated with less radial artery occlusion (RAO) and arm dysfunction. In the case of total RAO within the anatomical snuffbox, antegrade blood flow is assumed to be preserved through the superficial palmar arch with dTRA, thereby minimising the risk of thrombosis and extensive forearm RAO. TRA greatly reduces complications versus TFA, and there is no mortality risk, with the more at-risk the patient, intense the anticoagulation, or acute the procedure, the greater the benefit.7 Key publications in this field include the rationale and anatomic bases for dTRA by Sgueglia et al.8 and ultrasound guidance for dTRA by Hadjivassiliou et al.9
Addressing Low Rates of Adoption of a Distal Transradial Approach
Possible reasons cited by clinicians for low rates of adoption of dTRA include the learning curve, less than perfect haemostasis, and no perceived advantage compared with other vascular access approaches. To increase uptake of this approach requires a willingness to learn, acceptance of new techniques and procedures to complete familiar tasks, structured training and retraining, and practice. In addition, it is essential to consider the potential clinical and economic benefits of dTRA.
Lower Occlusion Rates with a Distal Transradial Approach Versus Conventional Access
A major benefit of dTRA is that puncturing occurs distal to the superficial branch of the palmar arch; therefore, any occlusion to the RA is unlikely to extend past the first branching point, where there is inflow. In contrast, conventional access can be associated with occlusion of the entire length of the RA, as there is no branching point until the site of radial origin. The occlusion rate is much lower with dTRA compared with conventional access,10 and any occlusion is unlikely to affect the whole vessel, thereby maintaining a site for further radial access.
Economic Considerations
A transradial approach has the potential to generate financial savings for institutions and healthcare systems.11 Transradial intervention and same-day discharge after elective PCI was shown to be independently associated with lower in-hospital costs than transfemoral intervention with overnight stay.12 For example, if 30% of eligible coronary interventions in the USA were switched from transfemoral intervention with overnight stay to transradial intervention and same-day discharge, the potential annual savings could be approximately 300 million USD.12
VASCULAR ULTRASOUND
Ultrasound Is a Critical Tool for Guided Access
Vascular ultrasound is a critical tool for real-time guidance of needle advancement to minimise arterial back-wall punctures (i.e., to enable single-wall puncture rather than through-and-through puncture) and venepunctures. It can also be used to identify vessel course, and to distinguish diseased and calcified arterial segments. Utilising ultrasound decreases the number of unsuccessful access attempts, thereby improving the accuracy and speed of access. Other advantages of ultrasound-guided access include less vessel spasm; vessel evaluation (e.g., for tortuosity, including radial loops) before, during, and after the procedure; vessel sizing; and troubleshooting.
Ultrasound is also beneficial in proximal radial access for procedures such as puncturing an impalpable RA (e.g., if the RA is small) or a pulseless RA (e.g., following bypass surgery in persistent shock-resistant defibrillation), and puncturing an occluded RA, for which haemostasis can still be achieved provided access is distal to the portion of the RA that dives under the brachial radialis.
A study of transradial approach PCI without ultrasound guidance published in 2009 indicated a total failure rate of 4.6%, for reasons including unsuccessful arterial puncture (13%), and RA spasm (34%), which was probably due to multiple blind access attempts, and the use of sheaths that were too large for the vessel.13 Guided access with ultrasound may eliminate many of these problems.
Uptake of Ultrasound-Guided Access Among Healthcare Professionals
Despite all these advantages, and no obvious potential disadvantages, uptake of ultrasound-guided access outside radiology remains sporadic, perhaps because of the initial cost, learning curve, and training requirements. This technique also initially adds time to the procedure, but routine use has been shown to reduce the time to femoral14 and radial15 access.
DISTAL TRANSRADIAL ACCESS AND ULTRASOUND TECHNIQUES
Anatomical Snuffbox Access
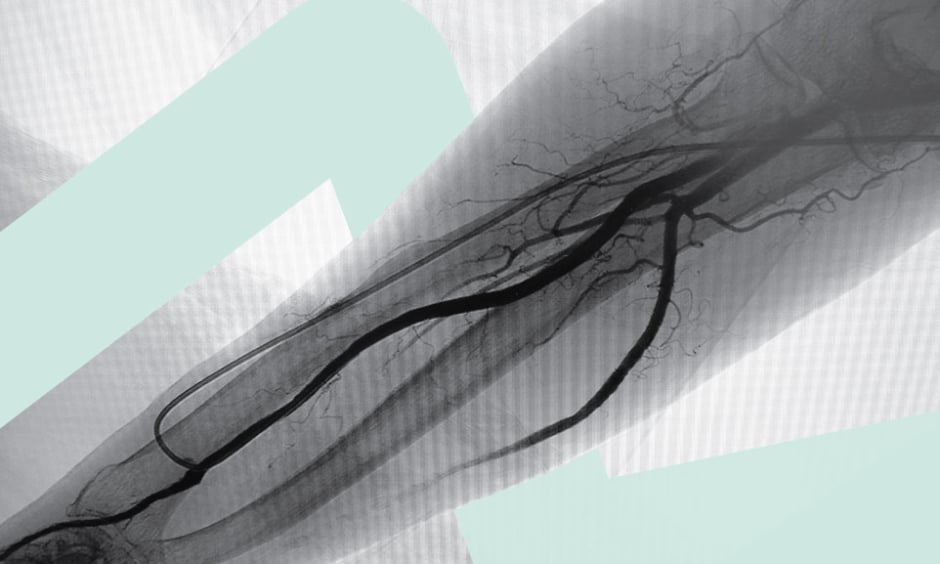
Three structures that need to be identified for snuffbox access are the dRA (a pulsatile vessel), the extensor tendons, and the radial nerve. It is important to avoid puncturing the extensor tendons, as this is painful for the patient, and may lead to tenosynovitis. Before attempting access, a thorough ultrasound assessment of the RA should be conducted, from the interosseus muscles to the radial head. To locate the snuffbox, start in the interosseus space between the thumb metacarpal and index finger metacarpal, and work proximally, looking for bones underneath the RA. Klass recommends: “Look for the artery between the two mountains.” Continue proximally to the thumb metacarpal, then to the trapezium and scaphoid. Puncturing the RA over the flat portion of these bones is ideal, taking care to avoid puncturing the cephalic vein, and rapid haemostasis can be achieved.9
To perform the procedure, a stainless steel wire (spring-coiled wires are too soft), local anaesthetic (2–3 mL) injected into the snuffbox, and a steep, single-wall puncture of approximately 60° are recommended. There is no need to nick the skin. After access is achieved, two movements are required to ensure the wire tracks through the vessel (for example, if there is bleed-back but the wire is not advancing): flattening the angle of the needle (spinning the bevel, if necessary), and angling the tip of the needle towards the volar side of the wrist. The average change in calibre from the RA to the dRA is approximately 0.2 mm,16 which means there is no significant loss of vessel size when puncturing at the snuffbox compared to conventional access, and there is no need to downsize the sheath when moving from proximal to distal.
Maximising Ultrasound Image Quality
For good resolution, a high-frequency ultrasound transducer (e.g., 10–18 mHz) is required, and a flat or linear transducer rather than a curved transducer provides the ideal versatility for different types of vascular access. In contrast, a hockey stick probe is limited to distal or conventional radial access. To achieve the best possible image, apply a generous amount of water-soluble sterile gel to the transducer, ensuring that there is no air between the transducer and the skin.
To stabilise the transducer and improve image quality, the operator can rest the transducer on the patient’s skin while placing their little finger on the heel of the patient’s imaging hand without applying any pressure. The orientation of the transducer can be established by running a finger along its edge while looking at the screen. Adjusting the gain (brightness of image) for near field and focusing on the middle of the RA also improves image quality.
Visibility of the Needle
A critical aspect of ultrasound-guided access is to know exactly where the needle tip is at all times. Two probe movements at the access point enable a clearer view of the artery and the needle, without physically moving the transducer. These movements are known as ‘heel-toe’, which involves angling the probe along its long axis, and ‘toggle’, which comprises rocking the probe along its short axis. Compression (z axis) also enables differentiation between vessels, as considerably more force is needed to compress an artery than a vein, and identification of vessel occlusion. If the transducer is vertical and the needle is at an angle, this impacts the visibility of the needle. The closer the needle is to horizontal, the clearer the image; hence, there is a trade-off between image quality and ease of access.
TIPS FOR DISTAL TRANSRADIAL ACCESS
Radial Cocktail
Radial cocktail is essential to reduce the risk of vessel spasm or occlusion. It should be administered slowly to minimise patient discomfort. Common elements of radial cocktail include nitroglycerin (nicardipine can be used but is expensive); unfractionated heparin (or bivalirudin); and a buffer typically made with 10–15 mL blood rather than saline, which is slightly acidic, and may lead to patient discomfort on administration of the cocktail. Verapamil or diltiazem persist systemically, and are no longer commonly used.
The onset of peak sedation and, occasionally, a vagal response to sheath insertion often coincide with the time of cocktail administration. Therefore, once the sheath has been inserted and secured, the pressure should be checked, and the cocktail administered only if the pressure is optimal, or administration should be delayed until vagal tone has resolved. If there is likely to be difficulty with catheter advancement or seating, initial administration of vasodilator only through the sheath should be considered, in which case, anticoagulant can also be given systemically. In the setting of RA spasm noted on angiogram, or if there will be multiple catheter exchanges, repeat doses of intra-atrial nitroglycerin and/or a warm pack to the forearm are recommended.
Recognising and Navigating Radial Loops
Radial loops occur in <5% of cases,17,18 and in all those cases, the recurrent RA arises from the apex of the radial loop (at the 12 o’clock position). When a wire is passed through a radial loop it will go straight up the recurrent RA every time, leading to vessel spasms and a trapped catheter, as the vessel is tiny. In the case of a radial loop on ultrasound, the recommended steps are to remove the wire, conduct an angiogram, use a hydrophilic wire or microwire to open up the radial loop, or, if needed, use a hydrophilic catheter and a stiffer wire to open up the loop. It is crucial to open up the radial loop to avoid any significant issues that could arise if the wire enters the recurrent RA.
Arm Preparation
For interventions where there is a need to move the patient’s arm, such as for cone-beam CT, the whole arm should be prepped to the axilla, then the arm mobilised and positioned using either a fenestrated drape or an arm board. A glove should be placed on the hand, and a U-shaped cut made into the glove at the snuffbox to allow the catheter or wire to pass through.
Sheath Selection
To achieve optimal access at the snuffbox, it is recommended to use a reinforced sheath, as non-reinforced sheaths are more prone to kinking. In cases where there is a radial loop, a longer sheath (e.g., 23 cm) can be helpful to avoid multiple passes across the loop. In cases of repeated RA access, or when dealing with small or spasm-prone vessels, the lowest profile sheath possible is recommended.
Radial Angiogram
For radial angiogram, it is recommended to use 3 mL dye at the time of access to visualise the size, route, angulation, and calcification of the vessel.
Access in Cases of Radial Artery Occlusion
A significant benefit with dTRA is that it enables access to, and navigation through, occluded vessels. In cases of RAO, the operator should access as normal, screen the wire as far as it will go, and ensure a stiff wire across the wrist joint for sheath insertion.
Normal Versus Abnormal Radial Artery
As the RA is a superficial vessel, any small haematoma will be visible on ultrasound. It is important to be familiar with the usual condition of the vessel so that any new post-procedure haemotomas, or other changes, can be identified correctly.
ADDRESSING CHALLENGES IN TRANSRADIAL INTERVENTION
Challenges in Radial Artery Access
If the RA has a faint pulse, is impalpable, or is difficult to access, the operator should consider whether the RA is absent, thrombosed, severely diseased, or calcified; the patient is hypotensive or on vasopressors; whether there are bony or vascular anomalies; or the RA is present but deep (e.g., in patients with morbid obesity). In challenging cases, the following strategies may be followed: performing a reverse Barbeau test to assess patency; using ultrasound with a vascular probe; subcutaneous infiltration of nitroglycerin; assessment higher in the forearm; or consideration of the ipsilateral ulnar artery (UA) or contralateral RA. If puncturing the skin is difficult, a micropuncture sheath can be used to create a track, and the sheath can then be inserted.
Slender Radial Approach for Small Vessel Access
The slender radial approach is a well-developed concept, derived from studies in Japan,19 and replicated in the USA.20 This approach involves using the smallest possible puncture, sheaths, and guides to perform technically adequate, complex radial PCI to reduce patient discomfort and complications, and preserve radial patency. Patients with small stature, small calibre RAs on pre-TRA ultrasound, radial spasm despite vasodilators, or RA atherosclerosis or stenosis are eligible for a slender radial approach. Also eligible are patients requiring repeated procedures from a TRA approach, and those for whom a simultaneous proximal and distal radial approach is planned.
Achieving Patent Radial Haemostasis
Technical and technological advances are needed to facilitate increasingly complex intervention via the slender approach. However, there is a need for consistent RA preservation and predictable rapid radial haemostasis, particularly in the context of repeated RA access.
Achieving patent radial haemostasis is critically important. This can be achieved by applying an RA compression band to the site of sheath insertion and inflating to occlude radial flow, removing the sheath and rapidly deflating the band, then re-inflating it to 1–2 mL, and compressing the UA, which reduces risk of RAO,21 to ensure a blunted but pulsatile waveform. Protocols for patent haemostasis vary according to the haemostasis device, amount of air placed in the balloon, extent and type of anticoagulation, and sheath size.
Longer haemostasis time has been shown to be an independent predictor of RAO (odds ratio per additional hour: 1.070; 95% confidence interval [CI]: 1.008–1.136; p=0.03).22 Arguments in favour of a shorter haemostasis time include patient comfort and preference, rapid mobilisation and discharge, reduced RAO, and possible improvement in early hand function. Improved cost-effectiveness is also a consideration.11 Arguments against a shorter haemostasis time include risk of developing haematomas, post-discharge bleeding, and a possible increase in risk of RA pseudoaneurysms.
MANAGING RADIAL ACCESS COMPLICATIONS
Potential Complications with Radial Access
Potential procedural complications with radial access can include RA spasm, RAO, and catheter entrapment (Table 1). Post-procedural issues may include compartment syndrome, pseudoaneurysm, and in rare cases, avulsion of the RA. Hand dysfunction is a rare complication that has not been well documented in the literature.
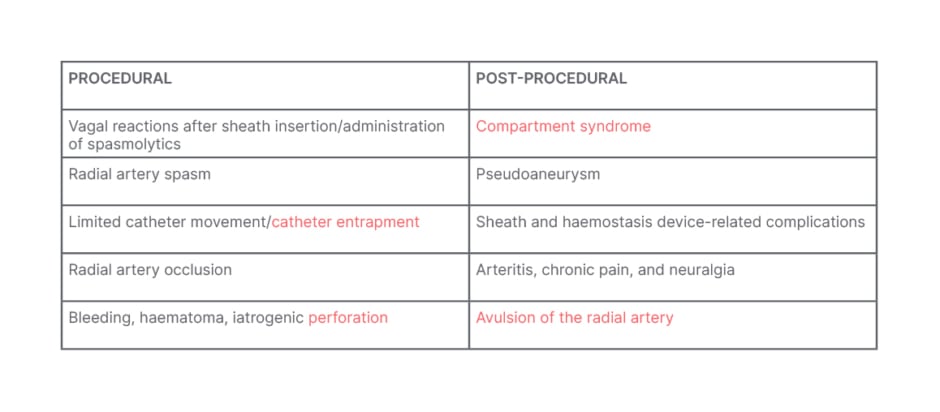
Table 1: Procedural and post-procedural complications with radial access.
Strategies to Manage Complications
Refractory radial spasm is uncommon, but can occur with larger catheters and more aggressive manipulation. Strategies to address this include using the lowest profile sheath possible, sedation, vasodilator dosing, and identification of anomalous vessels (e.g., radiocubital trunks).
RAO is the most common complication of radial access, and is often asymptomatic. Reasons for RAO include vessel overstretch, non-hydrophilic sheaths, failure to anticoagulate or achieve patent haemostasis, and excessive compression. Methods to reduce the risk of RAO include not oversizing sheaths and catheters, administering heparin (e.g., 50 IU/kg), UA compression, achieving patent haemostasis, and minimising repeat access. It is also important to check there is no thrombus in or at the tip of the sheath upon removal by aspirating to ensure patency.
Catheter entrapment may occur in the recurrent RA arising from the radial loop. Strategies for managing catheter entrapment are shown in Table 2.
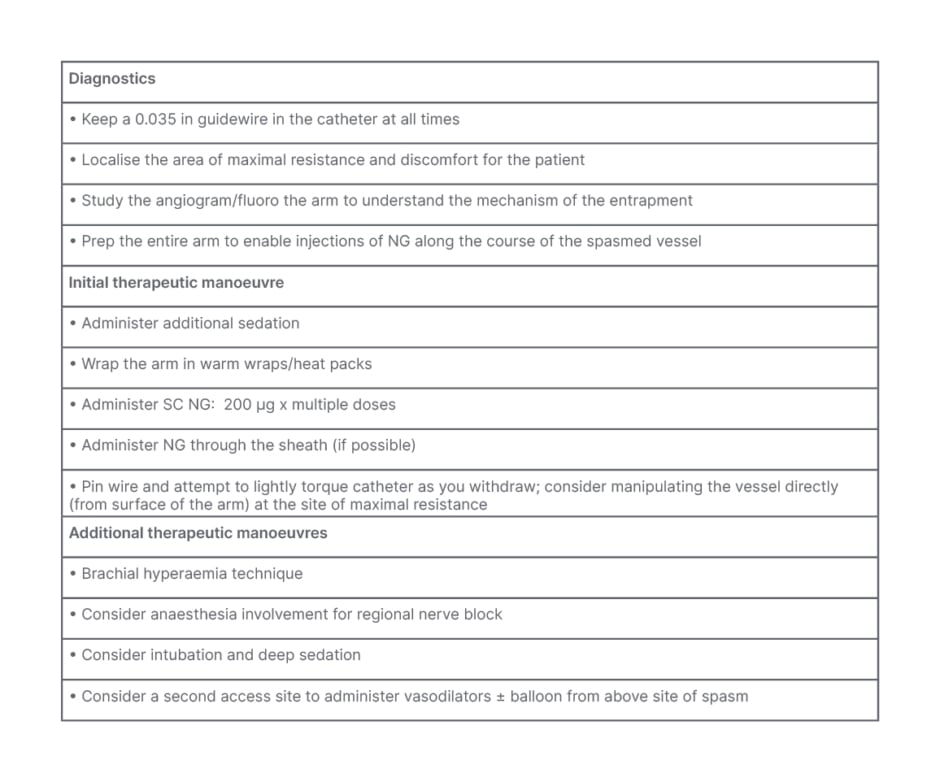
Table 2: Catheter entrapment treatment protocol.
NG: nitroglycerin; SC: subcutaneous.
Re-accessing the RA may lead to intimal hyperplasia,23 so a gentle approach is needed. In the event of a suspected radio-brachial perforation, it is advisable to take an angiogram, consider local compression (forearm) or manual blood pressure cuff (upper arm), and place a catheter across if tamponading the vessel. Pseudoaneurysms are best treated using compression.24,25 Neuralgia is an uncommon and self-limiting complication, and usually responds to non-steroidal anti-inflammatory drugs.
PERCUTANEOUS CORONARY INTERVENTION
Primary Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction
The most important technical considerations for primary PCI in ST-segment elevation myocardial infarction (STEMI) are timing, guide support, catheter calibre, and access or non-access site bleeding. Effective communication among healthcare team members regarding patient preparation, including preparation of bilateral femoral regions, and positioning, is also crucial to optimise ergonomics.
A meta-analysis of STEMI interventions showed that TRA was associated with better short-term survival and lower rates of major bleeding and vascular complications compared with TFA in patients with STEMI undergoing PCI.26 However, femoral rather than radial access should be used for patients undergoing chest compression.
Vascular ultrasound is useful in primary PCI for STEMI, as it decreases the time and the number of unsuccessful attempts; “the first poke is the best poke,” according to Nathan. Imaging-guided persistence is beneficial for access and catheter manipulation. If difficulty is encountered with wire or catheter passage, digital subtraction angiography of radioulnar anatomy is recommended. It is important to know when to accept access crossover in TRA in primary PCI; for example, in the case of a chronically occluded brachial artery, or a tethered and severely angulated RA.
Complex Percutaneous Coronary Intervention
Guide extension is essential for complex PCI. Most complex procedures and techniques are achievable with 6 French (Fr) guides, provided that there is effective guide support. Operators are encouraged to choose more guide support than they think is needed; familiarise themselves with adjunctive equipment, as well as guide engagement techniques and compatibilities; and be prepared for unexpected changes in equipment needs.
Radial to Peripheral
Scenarios in which to choose TRA for peripheral interventions include severe femoral arterial disease, morbid obesity, and tortuous, rotated, or previously stented aortoiliac bifurcations. dTRA is a useful option for radial to peripheral procedures.
RADIAL APPROACH IN INTERVENTIONAL RADIOLOGY
Operating Room Setup
Angling the patient’s arm at 45°, where space allows, helps maintain operator distance from the patient and reduces operator exposure to radiation. However, the cross body setup is not ideal for pelvic work.
Tips for Transradial Access in Interventional Radiology
Complex procedures using a TRA approach can be achieved by utilising coronary guide catheters, and requesting support and advice from interventional cardiologist colleagues. For interventions involving fibroid embolisation, pelvic intervention, or prostate embolisation, longer catheters may be necessary for taller patients, particularly when using dTRA. In cases of a tortuous aorta, access should be started with a coronary guide catheter rather than a diagnostic catheter. Advancing a coronary guide catheter with a diagnostic catheter through it along the RA is best achieved using a mother-and-child technique,27 to prevent the guide catheter catching on the RA and dissecting the intima. For example, a 5 Fr guide catheter with a 125 cm 4 Fr diagnostic catheter through it with 5 cm protruding will enable the guide catheter to move through the RA without damaging the vessel.
DISTAL RADIAL ARTERY ACCESS IN NEUROINTERVENTION
Recent Adoption of Radial Access in Neurointervention
Adoption of radial access in neurointervention is in its infancy. Most catheters currently used in this area come from femoral practice, rather than being radial-specific; therefore, there is some crossover to femoral access. In all cases, a patient-first approach rather than an access-first approach is preferable.
Preliminary Research with Transradial Access in Neurointervention
Preliminary research has shown that dTRA is a safe, effective, and promising approach for a variety of neurointerventions, and is associated with high patient satisfaction.28 A study incorporating a transradial approach in neuroendovascular procedures, including aneurysm coiling, stent- or balloon-assisted coiling, and stroke thrombectomy, showed that TRA was safe for both diagnostic and interventional procedures, with low rates of minor access site complications (0.6%) and crossover (5.2%).29
Lower access site and overall complication rates have also been shown with transradial versus transfemoral flow diverting stent placement to treat cerebral aneurysms (access site: 0.00% versus 2.48%; 95% CI: 2.40–2.57; p=0.039; and overall: 3.73%; 95% CI: 3.13–4.28 versus 9.02%; 95% CI: 8.15–9.89; p=0.035).30 Notably, one death resulted from a femoral access site complication in this study. Furthermore, a retrospective study showed that the dTRA approach enabled successful placement of flow diverters in patients with intracranial aneurysms with high technical success, no access site complications, one asymptomatic RAO (3.7%), and low femoral crossover rate (4.1%).31
Key Aspects of Transradial Access in Neurointervention
The use of ultrasound guidance and diagnostic angiography is key for dTRA in neurointervention. Redosing with radial cocktail without heparin before exchange, using a stiff guidewire and an arm board, securing buy-in from all healthcare professionals on the team, and being cognisant of inner and outer diameters of guide catheters are recommended. A learning curve of 5–50 cases, depending on experience, is to be expected.32 To enable more interventionalists to safely perform neurointerventional procedures via wrist access, catheter systems and devices specifically designed for radial access are needed.33
CONCLUSION
Transradial access is associated with reduced rates of bleeding, cardiovascular complications, and mortality compared with conventional access. dTRA with ultrasound guidance is a safe and effective option in specialties including interventional cardiology, interventional radiology, and neurointerventional radiology, and is associated with improved patient comfort and satisfaction. To increase uptake of this approach requires a willingness to learn, acceptance of new techniques and procedures to complete familiar tasks, structured training and retraining, and practice. Consideration of the potential clinical and economic benefits of dTRA is also important.
KEY QUESTIONS FROM THE Q&A SESSION
Who Is Most Likely to Develop Radial Spasm?
Age and sex are important factors in radial spasm, with young females being the most likely to develop this clinical problem. An adapted radial cocktail with increased amounts of verapamil and heparin may be used for young female patients, particularly those with fibroids. Concern surrounding the use of tissue-type plasminogen activator in these patients includes distal embolisation into the hand and associated sequelae. Heparin infusion appears to work well in these patients.
Regarding Radial Artery Occlusion, How Do You Conduct Ulnar Artery Compression?
To perform UA compression, wrap gauze tightly around the cap of a syringe and cover it with a transparent film dressing to create a cigar shape. Palpate for the UA, put the compression cigar on the UA, and then place the haemostasis device on top of the cigar. This temporarily occludes the UA, forcing blood through the RA, while getting haemostasis in the RA to achieve patent haemostasis. This reduces the risk of RAO. Remove the ulnar compression cigar when removing the haemostasis device.
How Valuable Is a Pre-procedure Barbeau Test?
If there is pre-procedure Barbeau D, choose another access site; however, pre-procedure Barbeau is not as reliable as once thought, does not correlate to outcomes, and is often not recorded.
Is There a Minimum Limit to the Radial Artery Size for Access?
This is case-dependent. One approach is there simply needs to be a pulsatile lumen on ultrasound for access to be attempted. Another approach is an absolute lower limit of 1.4 mm (pre-radial cocktail) with a 4 Fr sheath; and 1.7 mm with a 5 Fr sheath. Wrapping the arm in a warm towel may dilate the vessel. Clinicians who have no or little experience in radial access are recommended to work only with vessels greater than 2.0 mm in diameter until they are comfortable with the technique.
Are There Any Extra Measures for Patients Taking Direct-Acting Oral Anticoagulants to Avoid Pseudoaneurysm and Bleeding?
For patients taking direct-acting oral anticoagulants, or those with synthetic dysfunction, there may be a need to extend compression to 120 minutes, instead of the usual 90 minutes, to reduce the risk of pseudoaneurysm and bleeding.
What Is the Approach for Calcified or Diseased Arteries?
Ultrasound is critical. Try to find softer, less calcified areas in the artery for access. If pushing in the sheath is causing pain to the patient, leave the sheath protruding if necessary.