Abstract
Background:
Incidental findings on imaging are becoming increasingly common. An example of such a finding is a Vieussens’ arterial ring. It was first described by Raymond de Vieussens as a collateral pathway between the conus branch of the right coronary artery and the proximal right ventricular branch of the left anterior descending coronary artery. This finding can be of significance in patients with coronary artery disease.
Case Presentation:
The authors present the case of a 79-year-old male who experienced atrial tachyarrhythmia with a complex coronary artery fistula between the left anterior descending artery, conus branch, and the main pulmonary artery, indicating a Type Ib Vieussens’ arterial ring. Type Ib means that it is accompanied by vascular pathology. The patient also had additional coronary disease in the left anterior descending artery, further increasing the complexity of the case.
Conclusion:
Coronary collaterals offer an alternative source of blood supply in cases of additional coronary artery disease and can impact patient prognosis. Chronic myocardial ischaemia and increased shear stresses are causes of collateral circulations. Vieussens’ arterial rings can be subdivided into four groups. Only one case report of a Type Ib Vieussens’ ring has been identified on literature review, where the patient presented with a non-ST elevation myocardial infarction, unlike the atrial tachyarrhythmia in this case. Given their rarity, specifically of Type Ib, there is increased difficulty in establishing an appropriate management pathway, thus having great impact on clinical decision making.
Key Points
1. Coronary collaterals offer an alternative source of blood supply that can result in a prolonged period of myocardium viability in cases of original vessel failure or coronary occlusion.
2. Vieussens’ arterial rings (VAR) are embryological remnants that can act as collateral circulation, and the rarity of VARs can lead to difficulty in establishing the most appropriate management of patients presenting with it.
3. VAR Type Ib is described as a VAR that is associated with a vascular pathology, such as a fistula or an aneurysm, which is the case in the reported patient.
BACKGROUND
CT coronary angiography (CTCA) has been increasingly used in the diagnosis of coronary artery disease. Incidental findings on imaging are becoming more common in medical practice.1 The authors present a case of an incidental finding of a Vieussens’ arterial ring (VAR).
VARs were first described by French anatomist Raymond de Vieussens. They are observed in the population mostly as an embryonic conotruncal ring remnant;2 however, pathological VARs are very rare. It is widely described as a collateral pathway between the conus branch of the right coronary artery (RCA) and the proximal right ventricular branch of the left anterior descending coronary artery (LAD).3-5
This finding can be of significance in patients with coronary artery disease as it can be a means for cardiac perfusion. However, it is important to consider the implications of disease present in this collateral circulation when there is additional disease in the coronary arteries.6
CASE PRESENTATION
A 79-year-old male was admitted under the cardiology team with chest pain, shortness of breath, and palpitations. The patient reported a sudden onset of chest tightness, feeling clammy, and light-headed while walking 2 hours before presentation to their general practitioner. An ECG (Figure 1) performed by the patient’s general practitioner revealed narrow complex tachycardia at 150 bpm, which failed to respond to Valsalva manoeuvres, and paramedics were called.
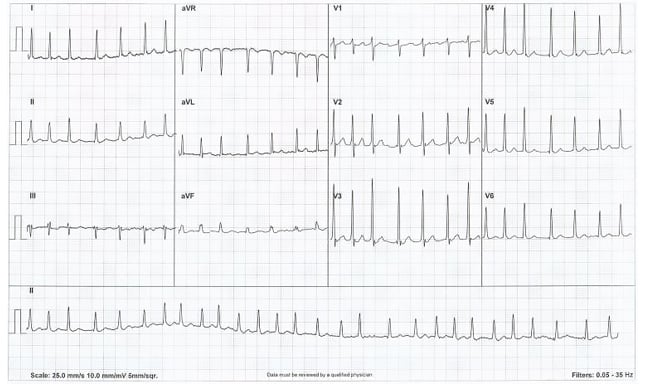
Figure 1: ECG demonstrating a narrow complex tachycardia.
Physical examination was normal; blood pressure was 147/84. The patient was transferred to the local district general hospital and whilst en-route, 8 hours after symptoms onset, the patient’s symptoms resolved, coinciding with self-cardioversion to normal sinus rhythm on the paramedic cardiac monitor.
No previous atrial tachyarrhythmias had been documented. However, the patient stated that a similar episode a few months ago had self-resolved. They had no significant past medical history and did not take any regular medication.
INVESTIGATIONS
Baseline investigations demonstrated normal full blood count, bone profile, renal profile, and thyroid function. Troponin I was elevated at 240 ng/L, but on repeat testing 3 hours later it had reduced to 117 ng/L (normal range: 0–34 ng/L).
Chest X-ray showed no abnormality. An ECG carried out at the emergency department, after ambulance crew handover, showed atrial fibrillation with a ventricular rate of 108 bpm.
A transthoracic echocardiogram to investigate possible aetiologies for atrial fibrillation revealed normal sized left and right ventricles with an ejection fraction of 55–65%, along with mild mitral regurgitation and mild-moderate tricuspid regurgitation.
Troponin elevation was likely rate related but a CTCA was arranged as an outpatient to screen for the presence of atherosclerosis. A diagnosis of Type 2 myocardial infarction due to atrial fibrillation with rapid ventricular rate was diagnosed. The patient was anticoagulated, given the new diagnosis of atrial fibrillation with a novel oral anticoagulant, and started on a β-blocker for rate control.
CTCA images (Figure 2) demonstrated a complicated lesion of the vessels arising from the anterior aspect of the mid-LAD traversing across the anterior aspect of the pulmonary artery outflow origin with a small associated aneurysm (1 cm). Thereafter, there was also a connection to the ostial RCA conus branch. There was a small connection between the aneurysmal portion and the pulmonary outflow tract. This would be classified as a Type Ib VAR variant.
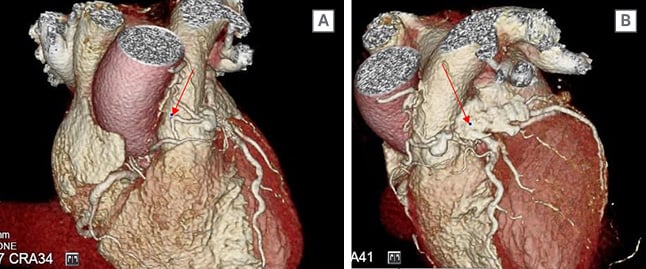
Figure 2: A CT coronary angiography demonstrated an unusual complex coronary artery fistula between the left anterior descending artery, conus branch, and the main pulmonary artery.
There was also associated moderate stenosis of the left anterior descending artery.
There were also two moderate stenoses in the LAD seen proximally, and a further possible moderate stenosis seen in the mid vessel. The circumflex demonstrated a calcified lesion in the mid vessel, which is remodelled with an underlying mild stenosis.
MANAGEMENT
The case was discussed at a local multidisciplinary imaging meeting and with a tertiary radiology team. The consensus opinion was that this abnormality was likely to be a prominent VAR that can be sub-defined as a Type Ib, which is associated with coronary disease and aneurysm/fistula.
A cardiac perfusion MRI was performed with adenosine, which demonstrated no significant late gadolinium enhancement (reflecting no significant previous infarct) and no inducible ischaemia (reflecting coronary circulation was satisfactory). It also demonstrated normal biventricular function. This case was rediscussed at interventional local multidisciplinary team meeting and consensus of outcome was for a conservative medical approach. A repeat ECG has been arranged to support decision making whether a further rhythm strategy is required if the patient has remained in atrial fibrillation.
DISCUSSION
Coronary collaterals are anastomotic connections, without an intervening capillary bed, between portions of the same coronary artery and between different coronary arteries.7 They offer an alternative source of blood supply when the original vessel fails and aid to reduce myocardial ischaemia during coronary occlusion. This may result in a prolonged period of myocardium viability following an acute myocardial infarction and, therefore, extends the period of time available until successful coronary reperfusion.8
Collateral circulation can develop as a consequence of chronic myocardial ischaemia stimulating biochemical signal release, including angiogenic growth factors and induction of the proliferation of smooth muscle cells, and an increase in shear stresses. In normal circumstances, the pressure in the right and left coronary systems is equal and there is no significant flow detected in the connection. However, when stenosis develops due to for instance atherosclerosis resulting in coronary artery disease, it results in the vessel dilating and allowing blood to flow.7
VARs are connections between the conus branch of the RCA and the proximal right ventricular branch of the LAD.4,9 They were first described in literature by French anatomist Raymond de Vieussens in 1706, who provided their name to an identified proximally epicardial connection between the RCA and LAD. Unlike ischaemia-induced collateral vessels, they are embryological remnants that become clinically significant as an intercoronary collateral vessels in the context of coronary artery disease.10
According to Dogan et al.,2 VARs can be subdivided into four variants, which are Ia: VAR with no accompanying vascular pathology (“classic VAR”); Ib: VAR with accompanying vascular pathology (aneurysm or fistula); II) VAR-like dual LAD duplication; and III) VAR with single coronary anomaly.
This is a rare case of a pathological VAR (Type Ib) and in Dogan et al.,’s2 evaluation of 3,443 consecutive CTCA, no cases of Type Ib were detected. Managing coronary artery fistulas and aneurysms is a complex and challenging process in itself, without the additional involvement of a VAR as an important variable in decision making. The authors would direct readers to the 2018 article ‘Management of coronary artery aneurysms’ for a comprehensive explanation of the decision making variables and processes involved when managing these findings.11 An internet literature review demonstrated only one case report of a Type Ib VAR in the context of a 55-year-old male presenting with non-ST elevation myocardial infarction, differing from the authors’ case of a presentation with atrial tachyarrhythmia.12 Given the rarity of this anatomical finding, there is no established guidance on approach to clinical management.







