Abstract
Transcatheter aortic valve replacement (TAVR) has been established as an alternative to surgical aortic valve replacement in select patients with severe aortic stenosis. Next-day discharge (NDD) after TAVR allow patients rapid mobilisation to return home. A minimalist pathway using NDD has been shown to be effective and safe in carefully selected patients. Following the COVID-19 pandemic and earlier reports of same-day discharge (SDD) after TAVR, in 2020 several institutions modified NDD protocols to carefully select patients for discharge the same day. These protocols maximised efficiency and resource utilisation while minimising COVID-19 exposures, hospital length of stay, and healthcare-associated costs, both to the institution and to the patient. In this literature review, the authors discuss the precedent for SDD after TAVR, investigate the pressure for efficiency amidst a global pandemic, and assess the safety and feasibility of SDD as seen across multiple healthcare systems.
NEXT-DAY DISCHARGE: THE CURRENT APPROACH
Transfemoral transcatheter aortic valve replacement (TAVR) is an alternative to surgical aortic valve replacement for most patients presenting with severe symptomatic aortic stenosis.1-6 Hospital stays historically ranged from 3–11 days with median length of stay (LOS) 4 days (2012–2015).7 In the 2020 Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) transcatheter valve therapy (TVT) Registry summary of 276,316 patients undergoing TAVR from 2011–2019, hospital stay had decreased to 2 days, with over 90% of patients discharged home.8 Crucially, hospital LOS has gained significant weight in the management of TAVR, as more bundled fixed payment systems have been implemented across healthcare systems. Several studies highlight the importance of streamlining the efficiency of TAVR with regards to expected cost burden reductions to the patient, as well as reduced overall in-hospital cost.3,4 Current research suggests next-day discharge (NDD) after TAVR reduces length of hospital stay, without an increased risk of complications.9 NDD has become more common, and over a quarter of patients are discharged 1 day after TAVR.
Consequently, over the last decade, ‘minimalist’ clinical pathways have been developed and implemented to facilitate safe discharge home at the earliest time after procedures. In 2019, the Vancouver Multidisciplinary, Multimodality, Minimalist (3M) Pathway for next-day discharge was validated using anatomic and functional screening criteria, along with peri- and post- procedure management guidelines to allow for NDD, while maintaining favourable safety and efficacy outcomes.10 The 3M Pathway is composed of a minimalist peri-procedure approach, facilitated post-procedure recovery, and criteria-driven discharge (Figure 1).10 Across 11 centres, 411 patients met the study inclusion criteria. 80.1 % of patients were discharged the next day per protocol, and all-cause mortality at 30 days was found to be 2.9% (95% confidence interval [CI]: 1.7%–5.1%). Secondary endpoints included a readmission rate of 9.2% within 30 days. The results from 3M were comparable to the two low-risk TAVR trials in the United States showing composite death from any cause at 1 year to be 1.0% and 2.3%, demonstrated favourable primary and secondary endpoint outcomes for patients discharged the day following TAVR, and establishing an evidence-based clinical pathway with excellent safety and efficacy outcomes.10-15
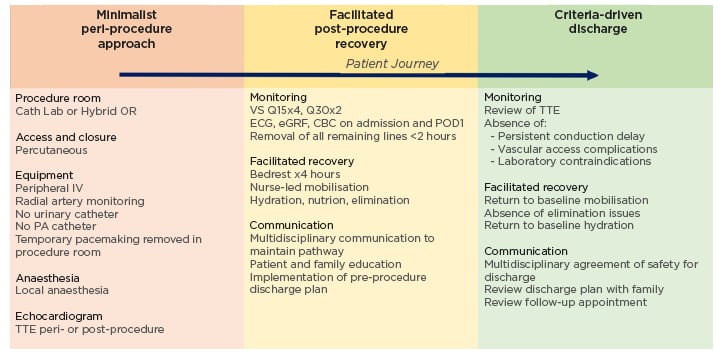
Figure 1: Vancouver multidisciplinary, multimodality, minimalist transcatheter aortic valve replacement clinical pathway.
Three components of the 3M TAVR Clinical Pathway: minimalist peri-procedure approach, facilitated post-procedure recovery, and criteria-driven discharge.
3M: multidisciplinary, multimodality minimalist; 3M TAVR: multidisciplinary, multimodality, minimalist transcatheter aortic valve replacement; CBC: complete blood count; eGFR: estimated glomerular filtration rate; IV: intravenous; OR: operating room; PA: pulmonary artery; POD1: post-operative Day 1; Q15: every 15 minutes; Q30: every 30 minutes; TTE: transthoracic echocardiogram; VS: vital signs.10
THE FIRST SAME-DAY DISCHARGE AFTER TRANSFEMORAL TRANSCATHETER AORTIC VALVE REPLACEMENT: SETTING THE PRECEDENT
In 2015, Généreux et al.16 published the first case of a 65-year-old male with severe aortic stenosis, New York Heart Association (NYHA) III symptoms of congestive heart failure, who was safely discharged home the day of transfemoral TAVR. The patient’s surgical history was significant for double coronary artery bypass grafting with percutaneous coronary intervention 10 years later. They presented with severe aortic stenosis (mean gradient of 46 mmHg, aortic valve area of 0.8 cm2, and left ventricular ejection fraction of 40%), and a calculated STS predicted risk of mortality (PROM) of 6%. Elective transfemoral TAVR with a balloon expandable Sapien XT (Edwards Lifesciences, Irvine, California, USA) was performed percutaneously under conscious sedation with minimal instrumentation. The procedure was uneventful; the total procedure time was 37 minutes. Post-procedural transthoracic echocardiogram showed a mean gradient of 5 mmHg, and aortic valve area of 1.9 cm2. Ambulation was allowed 6 hours after the procedure; telemetry showed no new conduction abnormality; complete blood count and electrocardiogram were comparable to pre-procedural. The patient was discharged home 10 hours post-procedure. The patient returned to normal daily activity on post-operative Day 2. No adverse events occurred during follow-up at 5-day and 30-day timepoints. This initial case demonstrated the possibility of same-day discharge (SDD) after TAVR, and of potential broader application to other similar patients.
SAME-DAY DISCHARGE AFTER TRANSFEMORAL TRANSCATHETER AORTIC VALVE REPLACEMENT: THE EXPERIENCE
As a result of the COVID-19 pandemic and the suspension of non-urgent surgical procedures, many institutions restricted access to elective cardiothoracic surgical and interventional cardiology procedures to reduce hospital admission and LOS, and limit both patient and healthcare worker exposure.13,17-27 Limited inpatient bed capacity, staffing shortages, and scarce resources necessitated an evolution in the delivery of care, including the addition of telemedicine and acceleration of traditional clinical care pathways. Coupled with patient hesitancy to seek care, these circumstances intensified the need for efficiency in time to treatment and to discharge. Considering the reductions in peri-procedural complications and a growing shift towards use of conscious sedation during TAVR, demonstrated in the 2020 TVT Registry Summary, a few institutions developed standardised clinical care pathways for SDD after TAVR, following existing NDD evidence-based protocols.10
Single-centre case series established the safety of SDD after TAVR.17-21,23,24,27 One of the earliest, from France, was published in 2020, demonstrating the safety of ambulatory TAVR in patients with pre-existing permanent pacemakers (PPM).17 This was followed by a similar series in the United Kingdom of 13 elderly patients with PPMs, who underwent TAVR via a ‘Daycase TAVR Protocol’, demonstrating no complications out to 30 days.18 Rai et al.19 described six patients without baseline PPMs who were discharged home the same day as transfemoral TAVR, but were monitored with a real-time remote heart rhythm monitor for 14 days. One patient with a new-onset left bundle branch block underwent additional electrophysiology testing demonstrating normal conduction, and was discharged the same day as the TAVR. No complications were reported during the follow-up period.Of note, patients considered for SDD met the following criteria: ambulatory, capable of performing activities of daily living, and robust social support.19 In the early experience with SDD after TAVR, remote monitoring was often utilised,19-21 but was not considered routine.16,23-27 By modifying the 3M protocol10,13 and implementing best practices of the NDD protocol,22 Pop et al.23 carefully selected patients for SDD after TAVR. Their protocol excluded patients with pre-existing bundle branch or atrioventricular block. They found no difference in the 30-day cardiovascular readmission rate for 29 highly selected patients discharged within the same day of TAVR. Moreover, at 30-day follow up there were no new PPMs implanted in the SDD after TAVR patients. They concluded there were no observable differences in safety outcomes compared to the standard NDD protocol, thus further supporting the feasibility of SDD.
Similarly, the Emory Heart and Vascular Center, Atlanta, Georgia, USA, created a SDD TAVR protocol, and published the outcomes in 2021.24 After careful evaluation by the Heart Team, every patient scheduled to undergo TAVR via a transfemoral approach under nurse-led conscious sedation was considered for SDD. Pre-specified characteristics making SDD unsafe or not feasible resulted in the patient being deferred to the NDD protocol. The exclusion criteria were divided into four categories corresponding to the phases of care: demographics, procedural variables, post-procedure, and discharge planning (Figure 2). A single-centre retrospective analysis was completed to evaluate the outcomes of 29 SDD patients after uncomplicated minimalist TAVR, as compared to 128 NDD prior patients identified via propensity matching, who would have qualified for SDD based on the standardised SDD clinical care protocol.24 Baseline demographic data was comparable between the two groups. Every patient in the SDD cohort was discharged on the day of their procedure after 6 hours of observation and meeting SDD criteria. No patients were discharged with remote monitoring. All-cause mortality at 30 days was zero in both cohorts, and interestingly, the rates of cardiovascular readmissions were higher in NDD cohort. Importantly, no SDD patient was readmitted with a new conduction abnormality or required a late pacemaker within 30 days.
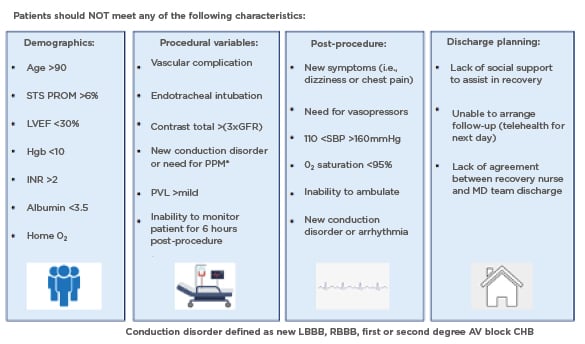
Figure 2: The Emory same day discharge protocol.20
Care pathway and protocol created to identify patients who could be safely discharged home the same day after uncomplicated, minimalist TAVR.
AV: atrioventricular; CHB: complete heart block; GFR: glomerular filtration rate; Hgb: haemoglobin; INR: international normalised ratio; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; O2: oxygen; MD: multidisciplinary; PPM: permanent pacemaker; PVL: paravalvular leak; RBBB: right bundle branch block; SBP: systolic blood pressure; STS PROM: Society of Thoracic Surgeons Predictive Risk of Mortality score; TAVR: transcatheter aortic valve replacement.
The multicentre PROTECT TAVR study, an international observational study of patients who underwent TAVR with SDD at seven sites, found SDD post-TAVR to be safe and feasible in select patients at low risk for clinical events post-discharge.25,26 Patient selection for SDD after TAVR was recommended by the local multidisciplinary heart team, but tended to follow an abbreviated 3M TAVR Clinical Pathway.10 Patients with pre-existing conduction abnormalities were excluded unless they had a permanent pacemaker. During the procedure, standardised minimalist TAVR best practices were followed: procedure performed in a hybrid room, only local anesthaesia and minimal sedations utilised, avoidance of central venous access and indwelling urinary catheters, ultrasound guided percutaneous vascular access and pre-closure of the large bore sheath site, and reversal of heparin with protamine at the conclusion of the procedure. Patients were monitored in the cardiac catheterisation recovery area for a minimum of 4 hours, and then mobilised. Standard post-procedure transthoracic echocardiogram and electrocardiogram were completed on every patient prior to discharge, and patients were discharged to their family after 6 hours if all SDD criteria were met. Complications were few, with no major vascular complications, strokes, or cardiovascular deaths out to 30 days. One patient received a pacemaker post-procedure, but was still discharged the same day. There were no cases of new conduction abnormality requiring a pacemaker from discharge to 30-day follow up. The composite of cardiovascular death, myocardial infarction, stroke, all-cause readmission, new permanent pacemaker implantation, and major vascular complications at 30 days occurred in only 5.7% of patients (driven by readmission of six of 106 patients: 5.7%) and readmission for cardiovascular reasons was 2.3%.26
Recently, Krishnaswamy et al.27 reported the Cleveland Clinic, Ohio, USA, experience with a SDD protocol compared to a NDD protocol for patients undergoing TAVR.27 Patients were candidates for SDD after TAVR if they met six criteria: transfemoral TAVR under conscious sedation; 6-hour post-TAVR bedrest with rhythm monitoring; no major complications or need for additional observation; stable haemodynamics and electrocardiogram; comfortable ambulation post-procedure; and post-discharge social support to assist in recovery.27 They too found low rates of complications for patients discharged on the day of the TAVR, with no deaths reported to 30 days. Notably, 5.3% of SDD patients developed a new left bundle branch block during the TAVR, all of which resolved during the observation period, and thus the patients were discharged later that same day. Predictors for successful SDD after TAVR included male sex, lower STS-PROM, and higher baseline haemoglobin level. Only seven of 114 SDD patients (6.1%) were readmitted within 30 days of the TAVR; notably, one patient was 103 years old, and two were admitted post-procedure Day 1 (one with a fever, and one with atrial fibrillation). Only one of the readmissions was for a new conduction abnormality that required implantation of a permanent pacemaker. The Cleveland Clinic protocol was less stringent, with no specific age or demographic exclusion criteria, than the Emory and PROTECT TAVR protocols, and may demonstrate SDD after TAVR is appropriate for a broader patient population.
THE CASE FOR SAME-DAY DISCHARGE AFTER TRANSFEMORAL TRANSCATHETER AORTIC VALVE REPLACEMENT: THE FUTURE
The safety of TAVR, along with the safety of NDD, have been well-established across a large spectrum of centres.8,10-15.22,28 With this in mind, we must consider the risks inherent to SDD, particularly the inability to immediately assess and provide appropriate care for a patient with post-procedural complications. Cardiac event monitors did not prove necessary, and were not routinely included in most institutional SDD protocols. Although one of the most common complications after TAVR is need for permanent pacemaker, this was extremely rare in the patients on the SDD pathway, highlighting the need for careful pre-operative patient selection.24-27 Moreover, late bleeding or vascular access site issues were also not demonstrated.
Careful selection of patients via evidence-based inclusion and exclusion criteria must be established to minimise the risks to patients after SDD. Universal criteria for SDD after TAVR have not been established, and institutional variability in these criteria has created ambiguity in appropriate selection of patients. Predictors for successful NDD after TAVR include male sex, young age, absence of atrial fibrillation, and lower serum creatinine.19 Further, consideration must be given to patient’s social support and geographic location relative to the home institution, as these factors may influence the risk-benefit ratio when choosing whether to safely discharge home on the same day of procedure.24,29
SDD after TAVR has emerged as a safe, efficient, and feasible option for carefully selected patients with symptomatic aortic stenosis, and limits the inpatient footprint and LOS. During COVID-19, SDD after TAVR ultimately led to improved resource utilisation, and a reduced nosocomial exposure risk to both patients and healthcare staff.20 While operating under the assumption that eliminating the overnight hospital stay is cost effective, additional research is needed to determine the true cost-saving impact of SDD TAVR. Recently, Cohen et al.30 presented the cost analysis of TAVR versus SAVR from the low-risk TAVR PARTNER 3 data at 2 years, and demonstrated overall cost was lower for TAVR with a saving of $2,030 per patient, with an average LOS of 3 days.30 Series assessing NDD have confirmed additional cost savings, and elimination of the inpatient stay may further improve cost effectiveness of TAVR. Consequently, SDD increased patient satisfaction, and enabled institutions to allocate resources justly amidst a global pandemic. The question remains if SDD TAVR will continue to be reimbursed, considering it is currently coded and billed as an inpatient procedure, thus requiring overnight admission.31 This poses a challenge to hospitals, and ultimately may require a significant shift in TAVR billing codes at the national level. A paradigm shift will further promote innovation that may benefit patients, hospitals, and the broader national healthcare system. Ultimately, additional studies are needed to assess the impact of technological advances and healthcare delivery in the expansion of SDD TAVR.







