Interview Summary
Cardiologists at four hospitals in Europe were interviewed on their experience with optimising the transcatheter aortic valve implantation (TAVI) patient pathway through implementing the Edwards Benchmark Program (Edwards Lifesciences, Irvine, California, USA) in their centres. Insights were received from José Díaz, Head of the Cardiology and Cardiovascular Surgery Department, Hospital Universitario Virgen Del Rocío, Seville, Spain; Franco De Remigis, Medical Director of the Haemodynamic Laboratory, Department of Cardiology, Hospital Giuseppe Mazzini, Teramo, Italy; Rajiv Das, Consultant Interventional Cardiologist, Freeman Hospital, Newcastle upon Tyne, UK; and Christophe Saint Etienne, Interventional Cardiologist, Centre Hospitalier Régional Universitaire (CHRU) de Tours, France.
Feedback from four European countries illustrates how a patient-focused approach to optimising the TAVI pathway has the potential to improve the quality of care across different healthcare systems, and in hospitals with varying sizes of multidisciplinary heart team. A number of themes emerged. All hospitals reported improvements in patient pathways, including an increase in the number of TAVI cases (e.g., by 25–100%) after implementing the programme; reported reduced length of hospital stay (e.g., 80–90% of patients discharged within 3 days, compared with 5–10 days before the programme), with improved patient satisfaction; and recommended the implementation of a patient pathway optimisation programme, like the Edwards Benchmark Program, in order to address increasing capacity issues, and improve the quality of care.
The interviews were conducted between April 2022–April 2023.
INTRODUCTION
Most patients with severe symptomatic aortic stenosis remain undiagnosed and untreated.1 Research has shown that better access to either transcatheter or surgical valve replacement can improve the prognosis and survival rates of patients with severe aortic stenosis, who would otherwise remain without treatment.2-4 Current European guidelines recognise the effectiveness and safety outcomes of TAVI for patients with severe aortic stenosis (e.g., Class IA indication for patients older than 75 years).5 Alongside this, TAVI has evolved over the past decade, becoming a safer and more efficient procedure.5-10
However, there is a strong clinical rationale for TAVI optimisation. In Europe, a rising demand for TAVI is exerting pressure on hospitals to increase their capacity for the procedure, but within currently available resources.5,11,12 Unfortunately, the current scenario is that many patients remain hospitalised after TAVI without an urgent medical reason, which not only increases capacity constraints, but can even negatively impact the health of those patients, by increasing the risk of falls or hospital-associated disability, for example.13-16 The COVID-19 pandemic in particular highlighted the advantages of an optimised TAVI patient pathway in clinical practice.17,18
THE EDWARDS BENCHMARK PROGRAM
The Edwards Benchmark Program is a global pathway optimisation programme focused on patients with TAVI. It was developed for all stages of the clinical pathway: pre-, peri-, and post-procedure. The programme aims to improve the quality of care for patients with TAVI, and the efficiency of heart teams, while optimised resource utilisation generates opportunities for increased capacity. Key elements are a multidisciplinary team approach; 10 evidence-based best practices, covering all stages of the patient with TAVI pathway; and peer-to-peer learning with programme faculty.
The 10 evidence-based TAVI best practices are detailed below.
Pre-procedure
Before the procedure:
1. a multidisciplinary heart team is involved in patient care, and attend regular meetings;
2. there is a standardised screening, assessment, and admission pathway; and
3. there is tailored education for patients and families, that includes shared decision-making, and discussion of post-procedure discharge plan.
Peri-procedure
During the procedure:
4. there are consistent clinical outcomes with appropriate patient safety targets for death, stroke, vascular complications, length of stay, permanent pacemaker, and 30-day cardiovascular readmissions;
5. there are optimised peri-procedure practices, including anaesthesia, staffing, and resources; and
6. there is procedure planning that incorporates lifetime disease management, and facilitates future transcatheter heart valve-in-transcatheter heart valve and coronary interventions.
Peri-procedure
After the procedure, there is:
7. standardised post-procedure care to facilitate nurse-led safe recovery and early mobilisation;
8. consistent management of conduction delays;
9. safe and timely discharge home; and
10. regular communication with referring physicians and programme administration.
Hospitals can choose the best practices they will focus on, based on self-assessment of their individual needs, and peer-to-peer support. Published data shows that the programme helps patients recover more quickly through earlier discharge home,10 improves quality of life across physical and mental health,7 and conserves healthcare resources,19 enabling multidisciplinary heart teams to treat more patients without any compromise on patient safety.
EARLY PATIENT MOBILISATION AFTER TRANSCATHETER AORTIC VALVE IMPLANTATION
All four cardiologists who were interviewed highlighted earlier patient mobilisation post-procedure as a key benefit of implementing the best practices in the programme. This is crucial for shortening patient stay and time in intensive care, and was achieved in elderly patient populations. “Early mobilisation is one of the main things that we will take from the [Edwards] Benchmark Program,” said Das. “We were able to mobilise the majority of our patients with TAVI within 4 hours of having the operation.”
REDUCED HOSPITAL LENGTH OF STAY
Reduced hospital length of stay was another benefit demonstrated across sites (Figure 1). In Seville, the team said that shortening patient stay was the most important change that the programme achieved. Previously, patients were discharged from hospital at 5 or 6 days after TAVI, but now approximately 60% of patients are discharged within 2 days. In Italy, length of hospital stay was reduced by up to 8 days. De Remigis said: “The greatest benefit of implementing the Edwards Benchmark Program, as far as our centre is concerned, has, above all, been the ability to reduce inpatient stays.” He added: “The cardiology team worked hard to reduce the length of time that patients spent in the intensive care unit (ICU), which is now 8–24 hours for 95% of patients with TAVI.” Before the programme, patients were transferred to the ICU for up to 30 hours.
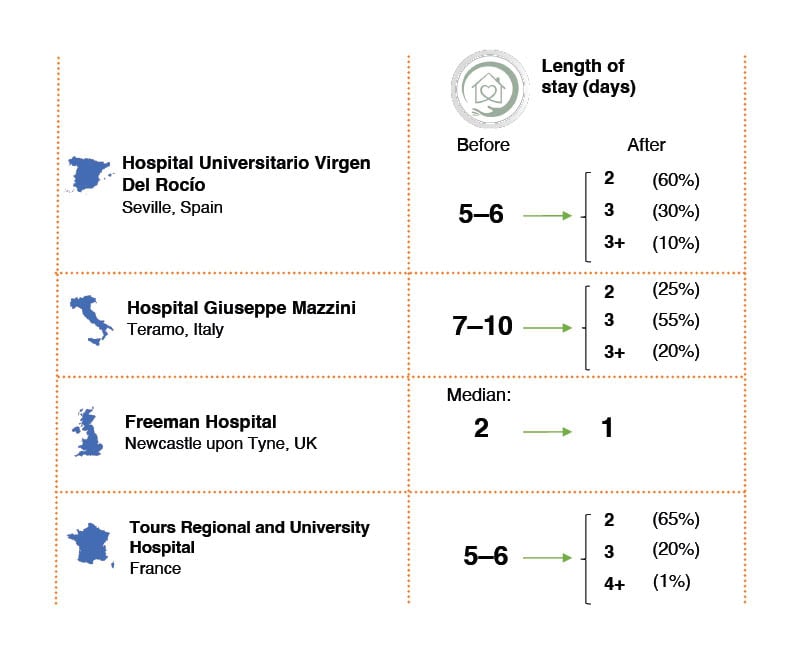
Figure 1: Real-life change in transcatheter aortic valve implantation length of stay in European
hospitals before and after implementing the Edwards Benchmark Program (Edwards Lifesciences, Irvine, California, USA).
Self-reported data by the hospitals.
In the UK, pre-discharge echocardiograms are no longer routinely performed in straightforward cases with good angiographic confirmation of valve position, which decreases time to discharge. Baseline echocardiograms can be done as an outpatient procedure. Most patients go home the day after the procedure, while very select patients are even able to go home the same day. Meanwhile, in France, more than 80% of patients are discharged by Day 3 post-procedure, with 40% discharged the day after the procedure. Importantly, the changes introduced by the university hospital team have not led to an increase in readmissions, demonstrating that earlier discharge, with reduced rates of pacemaker insertion, is safe.
HIGHER ACCESS TO TREATMENT, MORE EFFICIENT USE OF RESOURCES, AND COST REDUCTION
Reducing hospital length of stay can have a significant impact on daily inpatient costs, and allow faster turnover in wards, enabling more patients to be treated. In France, for example, the number of TAVI procedures completed per year has grown by approximately 25%, from approximately 400 to approximately 500, the equivalent of around 12 per week (based on 3–4 patients per day, 3 days a week). Meanwhile, in Spain, the number of patients has doubled from 2–4 each week. In Newcastle upon Tyne, the number of patients treated per day has increased from 3–4 to 4–5, and it is estimated that patients on the waiting list may be seen 25% earlier due to implementing the programme’s best practices. This is particularly important for patients with TAVI, who may deteriorate rapidly on a waiting list.20
Hospitals have also observed a reduction in the requirement for procedural equipment. Díaz said: “We found that the programme can reduce costs as it minimises invasive procedures, so fewer resources, such as pacemakers, are used.”
PEER-TO-PEER SUPPORT WAS FUNDAMENTAL TO SUCCESS
Evidence of the value and impact of the programme in other hospitals was important for reassuring heart teams that it would accomplish the desired objectives. During these discussions, faculty shared that the programme was successfully implemented at their own institution, and at other hospitals, through fostering open communication, collaboration, and support between peer groups, to guide the development and implementation of focused action points. This support was instrumental in easing residual doubts, and dealing with challenges along the way. In particular, there were concerns regarding the feasibility and safety of earlier discharge. Programme faculty shared previous experience, which showed that reducing the length of ICU stay was achievable, and helped heart teams to align on new management processes.
“Peer-to-peer faculty support was fundamental to our success, as we used their experience to convince the whole heart team to get involved, and to support us streamlining our TAVI procedures,” said De Remigis. “The programme protocols were challenging to implement, but this was overcome with the help of the programme faculty, and a consistent plan.”
A TRANSCATHETER AORTIC VALVE IMPLANTATION NURSE OR CLINICAL VALVE CO-ORDINATOR CAN BE CRUCIAL FOR OPTIMISING THE PATHWAY FOR PATIENTS
TAVI co-ordinators, also called clinical valve co-ordinators, provide a single point of contact for patients, and help to ensure that every stage of the pathway meets their individual needs, and that protocols for patient pathway optimisation are implemented. At the Freeman Hospital, the Edwards Benchmark Program helped the appointment of a clinical valve co-ordinator, who was instrumental in improving the efficiency of TAVI procedures within the department. Das said: “Having a specialist nurse (i.e., a clinical valve co-ordinator) was key to the success of our [Edwards] Benchmark Program. Her role was not just in co-ordination; a big part was patient education.”
At CHRU Tours, two TAVI co-ordinators have been at the heart of the programme. The hospital expanded their existing outpatient co-ordination role, so that they now follow the patient journey from start to finish, and build a trusting relationship with patients and their families. The clinical valve co-ordinators gain a comprehensive understanding of patients’ lifestyles, enabling them to develop personalised rehabilitation pathways following discharge, and increasing patient satisfaction with their treatment. The individualised process provides reassurance to patients and their families throughout the TAVI hospitalisation journey. “The patient is at the centre of the programme; they feel their management is holistic […] and they are all extremely satisfied with their TAVI hospitalisation experience. We’re winning on all fronts,” said Saint Etienne.
In keeping with these experiences, the Benchmark Registry found that more than 90% of patients were satisfied or very satisfied with the best practices implemented as a result of the programme. The results ranged from 90.3% for ‘informing family’ to 95.6% for ’respectful interaction’. Some 91.4%, 90.9%, and 90.8% were satisfied or very satisfied with the ’pre-TAVI discussion’, ’active participation in treatment decision’, and ’preparation for discharge’, respectively.21
BENCHMARK REGISTRY
The benefits of TAVI optimisation for patients and healthcare systems have been shown, not only in individual hospitals, but also in the ongoing Benchmark Registry.22,23 The multinational, multicentre, investigator-initiated observational study is being conducted in 28 centres in Austria, Czechia, France, Germany, Italy, Romania, and Spain.21 The analysis of the 30-day results included 2,405 patients, and was presented in a late-breaking clinical trial session during EuroPCR 2023. The results demonstrated significant improvements in efficiency of the patient with TAVI pathway, and patient safety was not compromised.
The first co-primary endpoint of the registry was overall length of hospital stay, which included door to TAVI and TAVI to door times (Figure 2).21 Prior to the Edwards Benchmark Program (n=882), the mean length of stay was 7.8 days. After programme implementation (n=1,425), the mean length of stay was reduced to 5.8 days, equivalent to a lowering of 2.0 days (p<0.001).
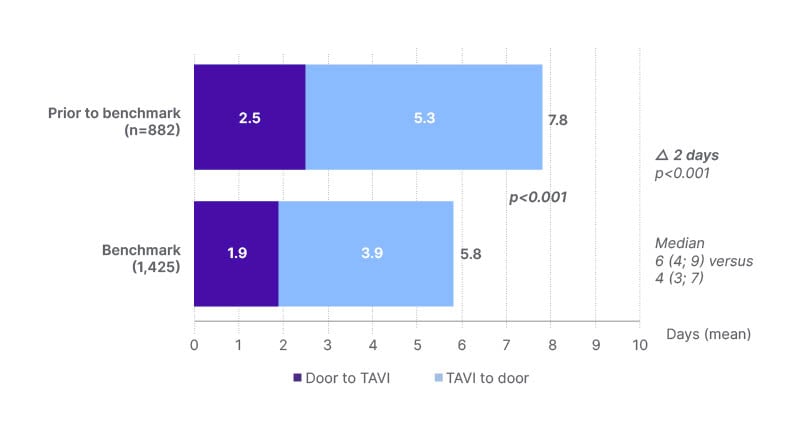
Figure 2: Hospital length of stay was reduced after implementation of Edwards Benchmark Program
(Edwards Lifesciences, Irvine, California, USA) best practices.21
TAVI: transcatheter aortic valve implantation.
The second co-primary endpoint was time spent in the ICU, cardiac care unit, and intermediate care unit, and prioritisation of a rapid return to a general ward. The average combined length of ICU/cardiac care unit/intermediate care unit/general ward stay was 5.3 days before (n=826) and 4.0 days after (n=1,351) programme implementation (p<0.001; Figure 3).21 The analysis also demonstrated a reduction in intensive care usage. The combined mean time in ICU/cardiac care unit/intermediate care unit reduced by more than 14 hours, from 1.9 days to 1.3 days, after implementing the programme’s best practices.
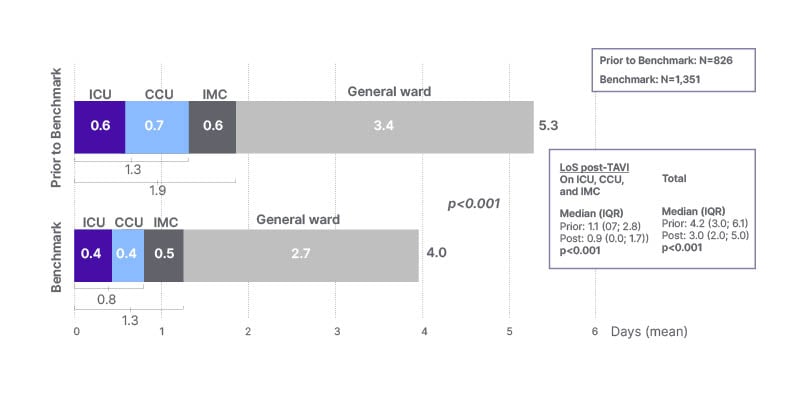
Figure 3: Intensive care unit length of stay was reduced after implementation of Edwards Benchmark Program (Edwards Lifesciences, Irvine, California, USA) best practices.21
CCU: cardiac care unit; ICU: intensive care unit; IMC: intermediate care unit; IQR: interquartile range; LoS: length of stay.
CONCLUSION
In order to treat the growing demand of patients diagnosed with severe aortic stenosis appropriately, hospitals all over Europe are under pressure to increase the numbers of TAVI procedures performed within existing resources. Across European healthcare systems, heart teams have observed that a patient-focused optimisation programme, addressing all steps of the TAVI pathway, promotes early patient mobilisation, reduces hospital length of stay, and makes more efficient use of resources, enabling more patients to be treated without reducing the quality or safety of care. A clinical valve co-ordinator can help to personalise the experience for patients and their families, increase the efficiency of logistics and communication, and make sure that the optimised patient pathway is implemented within the centre. All cardiologists interviewed recommended accessing Edwards Benchmark Program faculty support, and involving the entire heart team throughout the improvement process. As Díaz noted: “The skills, experience, and full engagement of the heart team at our hospital meant that the programme was implemented quickly and successfully.”
Saint Etienne concluded: “I think [the programme] has now improved our patient management, it has improved outcomes for our patients, and that has been done whilst also improving our patient satisfaction. Continuous improvement of patient management has to be a basic principle for every doctor. So, we will continue to improve our patient care, thanks to the Edwards Benchmark Program.”







