Abstract
The long-term complications associated with stent implantation for the treatment of coronary and peripheral artery disease have prompted a search for more conservative treatments, and a ‘leave nothing behind’ strategy. Drug-coated balloons are an attractive alternative that combine the advantages of balloon angioplasty with inhibition of neointimal proliferation and restenosis. Paclitaxel has so far been the drug of choice in balloon coating, given its high lipophilicity and local tissue retention. Still, its use is limited by a narrow therapeutic window and safety concerns. Sirolimus-coated balloons entered the drug-coated balloon arena late because of the need to use specific technologies to overcome pharmacokinetic limitations. Their use was initially tested in in-stent restenosis and small-calibre native vessels, demonstrating results that overlapped with those obtained with paclitaxel-coated balloons in terms of efficacy. New indications for sirolimus-coated balloon angioplasty are emerging, such as acute coronary syndromes, coronary bifurcations, peripheral and coronary medium- to large-calibre native vessels, critical limb ischaemia, vasculogenic erectile dysfunction, and dysfunctional arteriovenous fistulas. Data in these areas are still limited to small, non-randomised studies, showing encouraging results.
INTRODUCTION
Plain old balloon angioplasty (POBA) paved the way for percutaneous coronary treatment, and represented the beginning of modern interventional cardiology. Subsequently, coronary stents were initially introduced as a bailout strategy for complications associated with POBA (mainly acute recoil and flow-limiting dissection), and dual antiplatelet therapy represents the gold standard in treating coronary artery disease to the present day. Drug-eluting stents (DES) have shown to be more effective in the prevention of restenosis and repeated revascularisation than bare metal stents.1,2 Nevertheless, the events of late thrombosis or late stent fracture and cases of restenosis observed with DES represent the main limitations in their use.3,4 Hence, the attractiveness of drug-coated balloons (DCB) allows metal-free angioplasty, and limits barotrauma-induced intimal hyperplasia by delivering an antiproliferative drug that remains in the vessel wall for a limited time. Improvements in device characteristics and procedural techniques have limited acute complications related to balloon angioplasty in the use of DCB, which in any case can be treated with a stent bail-out strategy. The pharmacodynamic and pharmacokinetic properties of cytotoxic agents (taxanes), which are more favorable than those of cytostatic agents (limus) as an antiproliferative agent used in this technology,5 have led to the spread of paclitaxel-eluting balloon. In recent years, pharmacological limitations related to sirolimus and its analogs in DCB have been overcome by introducing specific balloon-coating technologies. Thus, as already happened for DES, and given some concerns about taxanes as an antiproliferative drug, recently limus drugs have been investigated for their use in DCB,6 both for the treatment of coronary and peripheral disease. At present, however, there is a lack of randomised data on their efficacy and safety profile, and comparison with taxane-eluting balloons and stents.
‘LEAVE NOTHING BEHIND’ STRATEGY
Improvements in coronary and peripheral stent design and the biocompatibility of the polymers and excipients used have reduced device-related cardiovascular events at midterm follow-up.7 In the long term, however, DES appears to be associated with cardiovascular event rates comparable to those of bare-metal sents (BMS),8 mainly related to the presence of an intravascular metallic device, which deters inflammation, intimal hyperplasia, and neoatherosclerosis. In addition, the duration of dual antiplatelet therapy can be reduced if a stent has not been released in the vessel, with benefit especially in patients at high bleeding risk. These considerations have led to incentives for developing ‘leave nothing behind’ strategies, such as the use of bioresorbable stents and DCB. After an initial setback related to the high rates of associated major cardiovascular events, the former has been completely modified in design and materials used, and still needs robust efficacy and safety data to allow a wide diffusion.9 On the contrary, DCB have shown promising results in many trials and clinical studies, with more consistent data available with paclitaxel-coated balloons (PCB). In recent years, however, data are emerging on the use of sirolimus as a balloon-delivered drug, whose wider therapeutic window compared with paclitaxel could represent an advantage in efficacy and safety, as has already been occurred with drug-eluting stents.10
TAXUS VERSUS LIMUS
The performance of DCB depends on the type of drug used, its morphology, dosage, and added excipients, but also on factors related to the lesion treated and patient characteristics. These factors interact in determining the concentration of drug released, and affect release kinetics and storage mechanisms.11
The two main pharmacologic classes used are taxanes and limus. Taxanes, of which paclitaxel is the most widely used, are cytotoxic drugs that interfere with the M phase (mitosis) of the cell cycle by stabilising polymerised microtubules, arresting cells at the G1 phase, and resulting in a proapoptotic effect. In contrast, rapamycin and its analogs exert a cytostatic effect by blocking the activation of a protein kinase critical in signal transduction (mTOR), and preventing the cell from moving from the G1 phase to the S phase of the cell cycle(Figure 1).12 The high lipophilicity with easy binding properties of paclitaxel has historically made it the drug of choice in DCB. However, paclitaxel is associated with high inflammation and toxic action at specific doses by cell apoptosis or necrosis,13 resulting in a narrower therapeutic window than sirolimus. In addition, sirolimus distributes equally throughout all vessel wall layers, unlike paclitaxel, which accumulates predominantly in the adventitia with a relatively low transmural diffusion gradient.5 To this should be added the recent meta-analysis by Katsanos et al.,14 which showed higher mortality in patients undergoing lower limb revascularisation and treated with PCB than those treated with POBA, with a more significant effect on mortality in those receiving the highest doses of the drug. Despite the conceptual and methodological limitations of the work,15 this evidence adds to previous findings showing lower mortality and superior clinical outcomes with everolimus-eluting stents compared with taxus.16 Therefore, in recent years, new technologies have been developed to overcome the pharmacological limitations of limus substances.
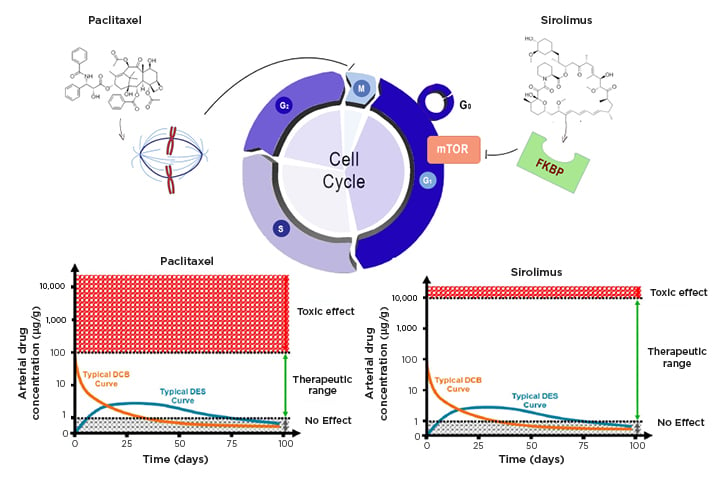
Figure 1: Effects of sirolimus and paclitaxel on cell cycle.
Paclitaxel and sirolimus act in two different phases of the cell cycle. The first interferes with microtubule organisation during cell division and blocks the cell in M phase. Sirolimus binds to the cytosolic protein FKBP and inhibits the mTOR pathway, which is involved in cell proliferation and the transition from the G1 to S phase of the cell cycle. The bottom graphs show the time course of drug concentrations in the arterial wall when released from a stent or balloon, and the different therapeutic windows of the two drugs.
DCB: drug-coated balloons; DEB: drug-eluting stents; FKNP: FK-binding protein; mTOR: mammalian target of rapamycin
Few devices with a sirolimus coating are currently commercially available. In particular, MagicTouch (Concept Medical, Gujarat, India) uses Nanolute technology, which delivers polymer-free nanocarriers containing sirolimus surrounded by encapsulation of a phospholipid excipient.17
Conversely, the micro-reservoirs of the SELUTION SLR™ (Med Alliance, Nyon, Switzerland) DCB, through the proprietary cell adherent technology (CAT™), are designed to provide controlled and sustained release of sirolimus to maintain therapeutic concentrations over a prolonged period of time.18 The Virtue® DCB (Caliber Therapeutics, New Hope, Pennsylvania, USA) is a microporous angioplasty balloon catheter carrying a sirolimus nanoparticle formulation. The drug is packaged with biodegradable polyester-based polymers.19 New SeQuent® Please (B. Braun, Melsungen, Germany) coated balloons (SCB) uses a sirolimus coating in crystalline form, and butylated hydroxytoluene as an additive (Table 1).20
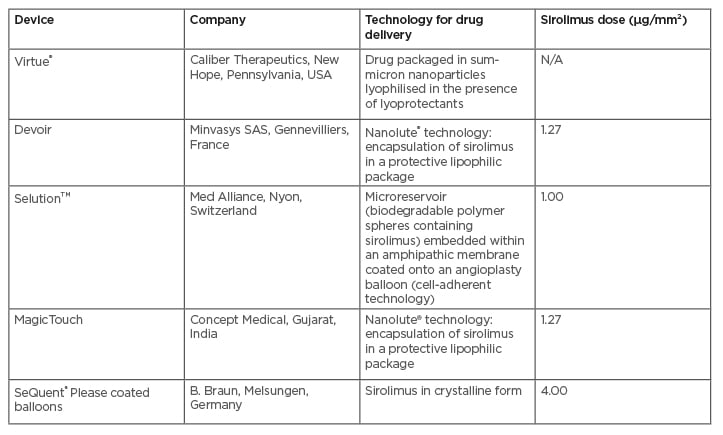
Table 1: Commercially available sirolimus-coated balloons.
N/A: not applicable.
TECHNICAL CONSIDERATION FOR DRUG-COATED BALLOON ANGIOPLASTY
The success of DCB angioplasty is influenced by factors related to the technical execution of the procedure, which includes preparing the lesion, performing proper dilation, and monitoring the result obtained.
The Third Report of the International DCB Consensus Group provides technical guidance on the correct performance of DCB angioplasty.21 In this setting, lesion preparation represents an essential step. Adequate pretreatment of the lesion allows better drug penetration into the vessel wall, and provides a better result in terms of lumen gain. Konishi et al.22 showed that a smaller area of residual plaque after pre-dilatation is associated with a lower rate of target lesion failure.
Pre-dilatation can be performed with semi-compliant or non-compliant balloons, while the use of specific devices may be helpful in the presence of calcific lesions. In such cases, calcium may constitute a mechanical obstacle to the diffusion of the drug, so a debulking with a cutting or scoring balloon or with other devices (atherectomy, laser) allows a better antiproliferative efficacy of the DCB angioplasty. A balloon-to-vessel ratio of 1/1 is recommended for optimal preparation of the lesion. In some cases, it is advisable to start with balloons 0.5 mm smaller than the reference diameter, such as complex anatomy or severe in-stent restenosis (ISR), to avoid balloon slippage. Aggressive dilation at high atmospheres using balloons with a balloon-to-vessel ratio of 1/1 is also helpful to correct any stent underexpansion that may have caused restenosis.23
Predilatation should be considered optimal in the absence of major dissection, thrombolysis in myocardial infarction (TIMI) flow <III, or residual stenosis >30%.21
Each DCB brand has specific instructions for proper dilatation performance. In general, patient transit time (i.e., time from balloon insertion in the introducer sheath to balloon inflation) should be short, to avoid excessive drug loss during delivery. The inflation time varies depending on the DCB used, but should not be less than 30 seconds. The length of the balloon should be such that it covers 2–3 mm upstream and downstream of the predilated area. The authors recommend inflating the balloon slowly to reduce vessel barotrauma, stable pressure maintenance for approximately 30–60 seconds to allow optimal drug transfer, and gently and slowly deflating to reduce vessel recoil. The absence of C-type or more severe dissection and a TIMI 3 flow downstream determine the success of angioplasty with DCB.21,23
SIROLIMUS-COATED BALLOONS IN CORONARY ARTERY DISEASE
Currently, the use of DCB is indicated primarily in the treatment of ISR and atherosclerosis of small-calibre native vessels (<2.75 mm or <3.00 mm). Acute coronary syndromes, bifurcation lesions, and stenosis in medium-to-large calibre vessels represent new frontiers in the use of DCB. Available data are predominantly from PCB, but the introduction of new technologies for coating balloons with sirolimus has led to a proliferation of preclinical and clinical studies using SCB (Table 2).
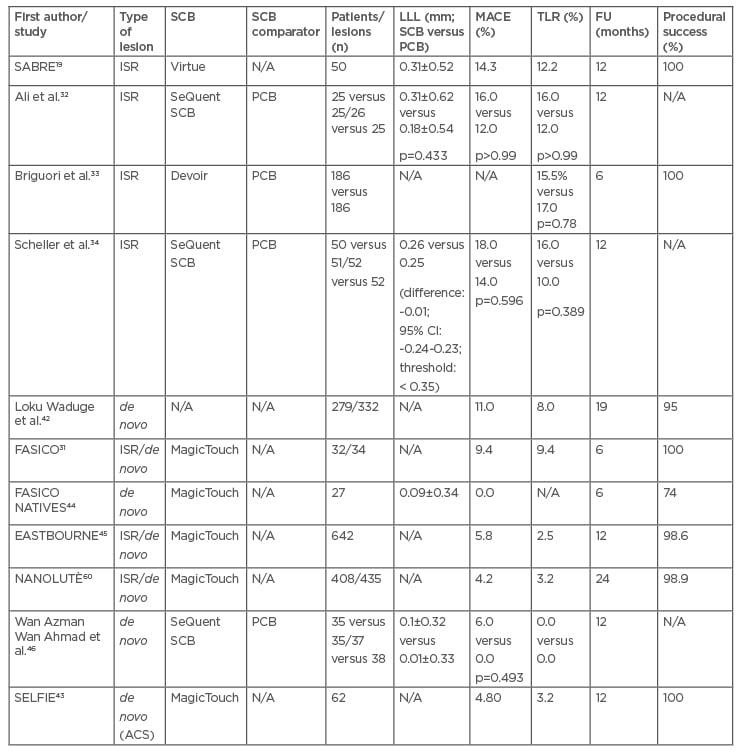
Table 2: Main clinical studies using sirolimus-coated balloons.
The table shows the main studies published to date in which SCBs have been used. Of these, only four have compared SCBs with PCBs and of these, most have used SeQuent SCBs. It can be seen that LLL and MACE tended to be higher in SRI studies than in studies with de novo lesions, although data variability is high. In addition, all studies have very high rates of treatment success.
ACS: acute coronary syndromes; FU: follow-up; ISR: in-stent restenosis; LLL: late lumen loss; MACE: major adverse cardiovascular events; N/A: not available; PCB: paclitaxel-coated balloon; SCB: sirolimus-coated balloon; TLR: target lesion revascularisation.
In-Stent Restenosis
In the case of ISR, a DCB angioplasty has the advantages of not adding a scaffold that could alter the anatomy of the vessel, preserving any collateral branches originating from the stenosed stent, and allowing a shorter dual antiplatelet therapy regimen.21 Histologically, ISR presents primarily as a phenomenon of intimal hyperplasia in BMS. On the other hand, in DES, it appears as a phenomenon of intimal hyperplasia associated with neoatherosclerosis. In the case of DES, if there are no mechanical problems (underexpansion, malapposition), the ISR phenomenon represents a failure to treat by antiproliferative drugs. The PACCOCATH ISR I24 and ISAR DESIRE 325 studies were the first to demonstrate the possible use of DCBs in ISR. A meta-analysis of 10 studies comparing second-generation DES and PCBs showed similar efficacy in treating ISR, with lower all-cause mortality in the DES group, which can be explained by differences between the two groups in observational studies.26 The DAEDALUS study pooled data from 10 randomised trials comparing angioplasty with PCB alone versus repeat stenting with DES alone to treat coronary ISR. At 3 years, repeat stenting with DES was shown to be moderately superior to angioplasty with DCB in reducing the need for target lesion revascularisation (hazard ratio: 1.32; 95% confidence interval: 1.02–1.70; p=0.035).27 A prespecified DAEDALUS analysis demonstrated similar efficacy and safety of DES and PCB in treating BMS-ISR, and a higher incidence of the safety endpoint (composite of all-cause death, myocardial infarction, or target lesion thrombosis at 3 years) in treating DES-ISR with PCB compared with repeat stenting.28
Currently, American guidelines do not consider the use of DCBs in the case of restenosis, but suggest the implantation of a new stent.29 In contrast, European guidelines consider in Class IA both the use of DES and DCB.30
Data on the treatment of ISR with sirolimus DCB are still scarce. The SABRE trial is a single-arm feasibility study of 50 patients with Virtue SCB showing good procedural success of using a sirolimus DCB to treat ISR.19 In the all-comer FASICO registry, 47% of indications for percutaneous coronary intervention (PCI) were ISR. MagicTouch SCB demonstrated 100% procedural success and excellent short-term efficacy and safety outcomes.31 Clinical trials to date compared SCB versus PCB in a limited number of patients, showing overlapping outcomes.32,33 During the Transcatheter Cardiovascular Therapeutics (TCT) conference 2021, Scheller presented data from two parallel trials (FIM Malaysian and German-Swiss) of SCB versus PCB to treat ISRA.34 SeQuent Please SCB proved non-inferior to PCBs in terms of angiographic late lumen loss at 6 months (0.30 mm versus 0.30 mm, difference 0; 95% confidence interval: -0.24–0.24; threshold <0.35).35
De Novo Coronary Lesions
DCBs are progressively emerging as a treatment for native vessels stenosis. In small-calibre vessels, PCI with stent implantation is limited by high restenosis rates and adverse outcomes.36
A series of non-randomised studies, registries, and randomised clinical trials have compared DCB with simple balloon angioplasty, BMS, and DES, with nonunique results. The PICCOLETO I trial37 randomised patients with stable or unstable angina undergoing PCI of small coronary vessels (≤2.75 mm) to Dior PCB or Taxus DES. The trial was stopped due to increased major adverse cardiovascular events in the DCB group, and demonstrated the importance of preparation, even in small arteries, of a stenotic lesion before treatment and effective formulation of the antiproliferative drug. The most recent randomised clinical trials, adequately designed (BASKET SMALL 2,38 PICCOLETO II39) have demonstrated the non-inferiority of PCB compared to stents. The results obtained in small-diameter vessels suggested using DCBs in de novo lesions in vessels >3 mm in calibre. The DEBUT study showed the efficacy of PCBs in treating de novo lesions in patients at high bleeding risk compared to BMS. In the study, 76% of PCBs used were >2.75 mm in diameter and 64% were >3 mm in diameter.40
The use of limus-coated balloons in native vessels is still in infancy. The BIO-RISE CHINA study showed the superiority of a biolimus-coated balloon over POBA in patients with small-vessel disease (reference vessel diameter <2.75 mm) for the primary endpoint of in-segment late lumen loss at 9 months.41 The use of SCB in small vessel disease has demonstrated promising results in a retrospective study with a mean follow-up of 19 months42 and a prospective registry.43 At the angiographic follow up at 6 months of the FASICO NATIVES registry, enrolling patients treated with MagicTouch SCB with a reference vessel diameter of 2.32 ± 0.44 mm, late lumen loss was 0.09 ± 0.34 mm, and the percentage diameter stenosis was 31 ± 18.44
EASTBOURNE is a multicentre registry designed to test the long-term safety and efficacy of SCBs (MagicTouch) in a real-world population. Reference vessel diameter was 2.58 ± 0.76 mm. In 55% of cases, these were de novo lesions, and analysis at 12 months showed good immediate performance and a good safety profile. Of note, SCBs give a higher rate of target lesion revascularisation in ISR than in de novo lesions (5.4 versus 0.2%; p=0.0008).45
Few data are available on a direct comparison between SCBs and PCBs in the treatment of de novo lesions.
Wan Azman Wan Ahmad et al.46 have recently presented data showing the non-inferiority of SCBs (SeQuent SCB, 4 μg/mm2) compared with PCBs (SeQuent Please Neo) in vessels ≥2.5 mm. Paclitaxel demonstrated, however, a more remarkable ability to determine positive remodeling (late lumen enlargement 58% in PCB versus 32% in SCB; p=0.019). These results have sparked debate about the possible different efficacy of limus and taxanes in the stentless treatment of de novo lesions.
The TRANSFORM I (TReAtmeNt of Small Coronary Vessels: MagicTouch Sirolimus Coated Balloon) trial comparing SCB versus PCB47 in small vessels (≤2.5 mm) and the TRANSFORM II (Sirolimus-coated Balloon Versus Drug-eluting Stent in Native Coronary Vessels) trial48 (in vessels with diameter >2.0 mm and ≤3.0 mm) are still ongoing, and will bring crucial results in the field.
Bifurcation Lesions
In treating coronary bifurcation lesions, a provisional single-stent approach is superior to systematic two-stent techniques.49 Use of PCB has been tested in the side branch with stent implantation in the main branch50 and in a stentless strategy,51,52 with good results of efficacy and safety.
Data with SCB are also limited in the treatment of bifurcations. Athulorala et al.53 and Jones et al.54 recently presented encouraging results of SCB use in the side-branch during provisional stenting technique in true bifurcations.
Acute Coronary Syndromes
The use of DCB has also been considered for the treatment of acute coronary syndromes. Sirolimus has demonstrated an essential role in reducing the degree of inflammation and migration of inflammatory cells, and stimulating the endothelium to the release of nitric oxide.55 The use of DCB in treating acute coronary syndrome, and especially in ST-segment elevation myocardial infarction, has a rationale for several reasons: patients are on average younger compared with those with chronic coronary syndromes, so the leave nothing behind strategy retains the possibility to intervene in different ways (coronary artery bypass graft, PCI with DES, or again with DCB) in case of progression of atherosclerosis or new acute events. In addition, the characteristics of the vulnerable plaques make them easy to treat with balloons, also allowing reduction in the duration of dual antiplatelet therapy.
Available data are mostly limited to PCB. After the negative results of DEB-AMI,56 some evidence has shown that a DCB-only strategy in the acute setting is safe and feasible, with good clinical and angiographic outcomes at medium-term follow-up.57–59 Randomised data on the use of SCBs in acute coronary syndromes are lacking. Data from the Nanolute60 and SELFIE registries43 show an excellent efficacy and safety profile of their use.
SIROLIMUS-COATED BALLOONS IN NON-CORONARY SITES
Sirolimus-Coated Balloons in Peripheral Artery Disease
Issues related to late complications of stent placement and the advantages of a leave nothing behind strategy have also been debated in the treatment of peripheral artery disease (PAD). DCBs have emerged as a new treatment option for obstructive PAD and critical limb ischaemia. European guidelines recommend using DCBs for ISR and short femoropopliteal lesions (i.e., <25 cm) as a Class B treatment option.61
As with coronary artery disease, the most consistent data are with PCB. Nine PCBs have been Conformité Européenne (CE)-marked for use in PAD, and three also have U.S. Food and Drug Administration (FDA) approval. Several randomised clinical trials have compared PCBs versus standard percutaneous transluminal angioplasty in PAD treatment, showing a superior efficacy and safety profile of PCBs compared with percutaneous transluminal angioplasty.62-66 Mixed results were reported in critical limb ischaemia treatment, a stage of PAD often under-represented in randomised clinical trials.67 Although registries and non-randomised studies have shown the use of DCB to be effective and safe,68 some concerns about possible distal embolisation of paclitaxel in an area already damaged by ischaemia, and reports of microvasculitis and panniculitis after treatment with PCB, have limited the use of this technology.69,70
On this background, interest in SCB has grown. At present, three SCB have been approved for the treatment of lower limb arterial disease: MagicTouch, SELUTION, and Virtue.
The former was tested in the prospective single-arm XTOSI study. Fifty patients with femoropopliteal or below-the-knee lesions were treated with SCB, with 100% technical and procedural success. The primary endpoint (6 month primary patency) was achieved in 80% of patients. At 12 months, freedom from clinically-driven target lesion revascularisation was 89.7%, and amputation-free survival was 81.6%, with no early safety concerns.71
The efficacy and safety of SELUTION SCB were evaluated in treating femoropopliteal lesions in 50 patients in the SELUTION SLR first-in-human trial. The mean late lumen loss was 0.29 ± 0.84 mm at 6 months follow-up, significantly lower than the 1.04 mm objective performance criterion value (p<0.001).72
The treatment with the device determined a rate of primary patency by duplex ultrasound of 88.4%, with a significant improvement in the Rutherford category73 (p<0.001), and in ankle brachial index measurements (p<0.001). Only one case of clinically-driven target lesion revascularisation was reported. In the prospective single-arm PRESTIGE study74, the same device was tested in below-the-knee lesions determining critical limb-threatening ischaemia in a population of 25 patients. Primary tibial patency at 6 months was 81.5%, with a technical success rate of 100%.74 All current SCB studies are limited by short- to medium-term follow-up.
Sirolimus-Coated Balloons in Erectile Dysfunction
Erectile dysfunction (ED) can have a vascular cause in 60–80% of cases when stenosis of the iliac-pudendal-penile arteries impairs perfusion of the male genital organ.75
Angioplasty with POBA is associated with recoil in a high percentage of patients,76 while a high rate of restenosis was observed after DES placement.77,78 The PERFECT-4 study79 enrolled 44 patients with ED and obstructive penile arterial lesions randomised to POBA or PCB angioplasty. There were no significant differences between the two treatments in the rate of restenosis at 8 months (40% versus 48%; p=0.569) and clinical success at 12 months (50% versus 59%; p=0.545), but both treatments were safe with no adverse events in the two groups. A meta-analysis on endovascular treatment of vasculogenic ED showed the procedure’s safety, and highlighted the heterogeneity of the results of the various included studies.79
The authors’ experience of DCB utilisation for endovascular treatment of ED comprises treatment of 194 consecutive patients with International Index of Erectile Function (IIEF-5) score <21, positive penile Doppler, and failure of drug treatment. Two hundred and thirty-eight lesions were treated, of which six (16%) were at the level of the internal iliac artery, 155 (65%) of the internal pudenda, 57 (30%) of the common penile artery, and 10 (4%) of the dorsal artery of the penis. The affected segment’s length was 11.9 ± 6.6 mm, with a vessel’s reference diameter of 2.2 ± 0.5 mm (minimum lumen diameter of 1.2 ± 0.6 mm). Relative stenosis was 73 ± 6.5%. A PCB was used in 141 lesions (59%), and SCB in 56 (24%). Procedural success (defined as residual stenosis <10% without signs of dissection) was 98%. Clinical success (defined by Δ IIEF-5 baseline score versus 3/6/8 months >5 points) occurred in 74.1% of patients treated with SCB, and 78.2% of patients treated with PCB (p=NS). At 8 months, clinical success was 68.9% in the SEB group, and 63.1% in the PCB group.80
The authors started to enroll patients with vasculogenic ED not responding to phosphodiesterase-5 inhibitors for >1 year and presenting with an IIEF-5 score <12, and a dynamic Doppler with Caverject® (Pfizer Inc., New York, USA) injection with peak systolic velocity <20 cm/sec in the multicentre, prospective, SUASION Registry.81 Angioplasty was performed with SCB. Of 27 patients enrolled, more than 74% had an improvement of >5 in the IIEF-5 score, and 73% had a Doppler peak systolic velocity score increase of >10. At 6 months, this was 70.4% and 68.4%, respectively. Procedural success was 100%, and in a minority of cases (14%), a drug-eluting stent was required.
Sirolimus-Coated Balloons in Carotid Disease
The use of DCBs has shown promising results in the treatment of carotid ISR82 where, however, experience with SCB is limited to a few cases described in the literature.83 Piccoli et al.84 used a pre-dilatation with PCBs before carotid stenting in patients with post-endarterectomy restenosis, demonstrating at a follow-up of 18 months no >50% restenosis, with only a transient ischaemic attack during DCB inflation, and one death during follow-up due to a myocardial infarction.84 Similar experiences are not described with SCBs.
Sirolimus-Coated Balloons in Dysfunctional Arteriovenous Fistulas
DCB was used in stenotic arteriovenous (AV) fistulas for dialysis. PCBs are superior to standard angioplasty in treating dysfunctional fistulas at 6 months.85-87 Angioplasty with SCBs is feasible and safe in treating dysfunctional or thrombosed AV fistulas with MagicTouch AVF SCBs.88,89 Based on the results of these two pilot studies, the IMPRESSION trial comparing DCB angioplasty with MagicTouch AVF SCB versus POBA in dysfunctional AV fistulas was designed.90 Recently, Tang et al.91 reported the 6 month and 12 month results of the ISABELLA registry, a prospective, single-arm study testing the feasibility and safety of SELUTION SLR SCB in the treatment of failing AV fistulas in 40 patients.91 Technical and procedural success was 100%, with no adverse events. Target lesion primary patency rate and circuit access patency rate at 6 months were 28/39 (71.8%) and 22/35 (62.9%), respectively, whereas at 12 months they decreased to 16/36 (44.4%) and 10/32 (31.3%), respectively. Among the interpretations provided by the authors to explain these results, there is probably an insufficient share of the eluted drug to allow a duration of long-term effects.
CONCLUSION AND FUTURE PERSPECTIVES
There is a familiar feeling among interventional cardiologists that sirolimus is better than paclitaxel as an antiproliferative drug used in stents or balloons. This perception stems from some safety considerations about paclitaxel and the action on vessel wall cells evident in preclinical studies.
The chemical and pharmacokinetic characteristics of sirolimus have limited its use and delayed its entry into the market, compared with paclitaxel as a DCB coating. This has resulted in far less efficacy and safety data on SCB than on DCB at present. Comparisons between the two types of DCB are limited to a few non-randomised data.32,92 Angioplasty with SCB appears to be effective and safe in all settings in which it has been tested, with short- and medium-term follow-up in most cases. Paclitaxel has demonstrated promising results in terms of late luminal enlargement as it reaches the tunica adventitia. In this sense, expectations are predominantly for long-term outcomes of SCB in treating native vessels. The TRANSFORM I47 and TRANSFORM II48 trials will help shed light on this point. Given the specific technologies in drug delivery of different SCBs on the market, it is also necessary to confirm the presence of a class effect and overlapping results of the various platforms.








