Abstract
Early discontinuation of dual antiplatelet therapy (DAPT) has been identified as a risk factor for late stent thrombosis after the implantation of drug-eluting stents (DES). Different durations of DAPT have been evaluated in observational studies and randomised controlled trials, but the results on the risk of ischaemic and bleeding events have been variable and controversial. Although extended DAPT shows an ischaemic benefit, it is associated with increased bleeding risk, while short-term DAPT has been suggested to be safe in recent trials with the newer generation of DES. Uncertainty regarding the optimal duration of DAPT makes clinical decisions challenging. In this review, evidence from the latest clinical trials on the duration of DAPT after DES implantation and the factors that affect DAPT duration is examined to find the optimal balance between thrombotic and bleeding risks, which would be a useful guide to clinical practice.
INTRODUCTION
Drug-eluting stents (DES) are implanted to reduce the likelihood of restenosis in percutaneous coronary intervention (PCI). Compared with bare- metal stents (BMS), DES significantly reduce the risk of restenosis and the need for target-lesion revascularisation. However, a DES is associated with increased late stent thrombosis (STH) and very late STH, which may result in life-threatening complications.1-4 Dual antiplatelet therapy (DAPT) means using aspirin together with a platelet P2Y12 receptor antagonist such as clopidogrel, prasugrel, and ticagrelor. This therapy reduces the frequency of STH in patients with BMS implantation effectively;5 therefore, DAPT is thought to be effective in reducing the risk of STH, myocardial infarction (MI), and subsequent ischaemic complications at sites outside of stented segments after DES implantation.
Currently, the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/Society for Cardiac Angiography and Interventions (SCAI) Guideline recommends that patients with stable ischaemic heart disease should receive 6 months DAPT instead of 12 months DAPT after DES implantation.6 The European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) recommend a 6-month regimen for patients with stable coronary artery disease after receiving new-generation DES.7 A 12-month regimen is still recommended for those with non-ST-segment elevation acute coronary syndrome (ACS) and ST-segment elevation myocardial infarction (STEMI) by both guidelines. However, the level of evidence for both recommended durations of DAPT is Grade B,7 which means more clinical studies are required to support these guidelines. Studies have been conducted to evaluate the safety and efficacy of different durations of DAPT recommended by different societies. Therefore, there is a need to review the currently available evidence on optimal durations of DAPT after DES implantation.
Premature cessation of DAPT has been identified as the most important risk factor of STH, which has led to recommendations for a prolonged DAPT regimen.8 However, previous evidence has suggested that extended DAPT is associated with increased risk of bleeding as well as all-cause mortality.9-12 With the recent advances in DES and the development of newer antiplatelet agents, determination of the optimal DAPT duration is crucial for balancing risks and benefits between ischaemic benefits and bleeding. The research question has become how to avoid premature discontinuation of DAPT and prevent increased bleeding at the same time.
CLINICAL EVIDENCE ON DURATIONS OF DUAL ANTIPLATELET THERAPY
Evidence from Observational Studies
Observational studies have shown the benefit of extended DAPT beyond 1 year in terms of lower mortality rate and the frequency of STH and MI. The characteristics of these studies are summarised in Table 1. The BASKET-LATE study reported a greater frequency of late cardiac death or MI in patients receiving DES implantation compared with those receiving BMS implantation (4.9% versus 1.3%) after the discontinuation of DAPT at 6 months.8 The Duke Heart Center Registry found a 50% increase in the rates of all-cause mortality or MI in patients in whom DAPT was withdrawn at 6 months compared with those who received 24 months DAPT (3.1% versus 7.2%, p=0.02).13 In the Dutch Registry, early discontinuation of DAPT after DES implantation was reported as a strong predictor of STH (30.7% of 418 DES receivers).14 Similarly, the Melbourne Interventional Group (MIG) Registry found a lower mortality rate in patients receiving 12 months DAPT than patients receiving 6 months DAPT (2.8% versus 5.3%, p=0.012).15 However, in the PARIS Registry, continuing DAPT beyond 12 months did not reduce thrombotic risk but was associated with a higher risk of major adverse events when compared to physician-guided cessation for stable patients, which therefore challenged the existing paradigms for extension of DAPT duration.16 In these studies, first-generation DES were largely used. Therefore, these observations may not be applicable to newer generations of DES.
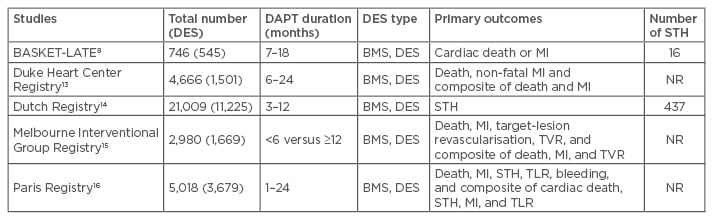
Table 1: Characteristics of observational studies allocating different dual antiplatelet therapy durations.
DAPT: dual antiplatelet therapy; MI: myocardial infarction; NR: not reported; STH: stent thrombosis; BMS: bare-metal stent; DES: drug-eluting stent; TVR: target-vessel revascularisation; TLR: target-lesion revascularisation.
Evidence from Randomised Controlled Trials
Two randomised controlled trials compared shortterm DAPT <12 months and extended DAPT for ≥12 months, while six trials compared safety and efficacy between short-term DAPT <12 months and 12 months DAPT. The characteristics of these studies are summarised in Table 2. All studies demonstrated the non-inferiority of shorter duration of DAPT (3–6 months) with comparable or even better efficacy and safety outcomes than longer duration of DAPT (≥12 months).
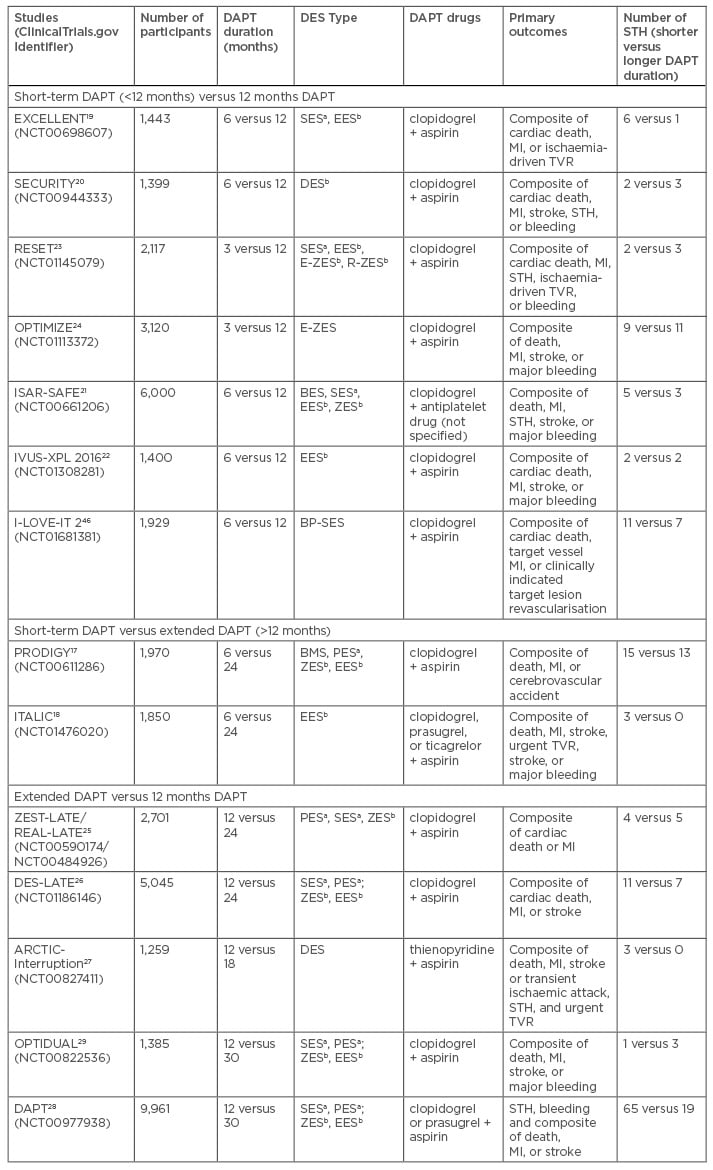
Table 2: Randomised controlled trials comparing 1) short-term DAPT (<12 months) and 12 months DAPT; 2) short-term DAPT and extended DAPT (>12 months); 3) extended DAPT and 12 months DAPT.
aFirst-generation DES: PES, SES; bSecond-generation DES: ZES, EES.
DAPT: dual antiplatelet therapy; MI: myocardial infarction; STH: stent thrombosis; TVR: target vessel
revascularisation; BMS: bare-metal stent; DES: drug-eluting stent; PES: paclitaxel-eluting stent; SES: sirolimus-eluting stent; BP-SES: biodegradable polymer sirolimus-eluting stent; ZES: zotarolimus-eluting stent; E-ZES: endeavor zotarolimus-eluting stent; R-ZES: resolute zotarolimus-eluting stent; BES: biolimus-eluting stent.
In the PRODIGY trial, patients were randomised to either three different types of DES, or BMS, while receiving 6 or 24 months DAPT. No significant difference was observed in the primary endpoints between the two groups during the follow-up, except for patients that received zotarolimus-eluting stent implantation. However, more frequent bleeding was found in the 24-month group.17 The ITALIC study demonstrated that the rates of bleeding and thrombotic events did not significantly differ between patients receiving these two DAPT durations after everolimus-eluting stent implantation.18
Both the EXCELLENT and the SECURITY trials suggested the non-inferiority of 6 months compared to 12 months DAPT regarding the incidence of cardiac death, MI, stroke, STH, and bleeding after either first-generation or second-generation DES implantation.19,20 In the ISAR-SAFE study, there was no significant difference in the prevention of death, STH, MI, stroke, and major bleeding between 6 months and 12 months DAPT,21 which was further confirmed in the recent IVUS-XPL trial.22 Randomised controlled trials with shorter DAPT of <6 months have also been conducted. The RESET trial showed that 3 months therapy was non-inferior to 12 months therapy with respect to the incidence of primary endpoints, which included cardiac death, MI, STH, ischaemia-driven target vessel revascularisation (TVR), and bleeding.23 This finding was also consistent with the OPTIMIZE study, which suggested that there were similar effects of 3-months and 12 months DAPT in reducing adverse clinical and cerebral events among patients receiving second-generation DES as no significant increase in STH risk was found in the 3-month group.24
There were four randomised controlled trials comparing safety and efficacy between DAPT beyond 12 months and 12 months DAPT. The characteristics of these trials are summarised in Table 2. All studies reported that extended DAPT was associated with increased frequency of major bleeds. Results from the ZEST-LATE/REAL-LATE trials showed the rates of composite events (MI, stroke, and death) and bleeds were higher in patients receiving 24 months DAPT.25 The extended DES-LATE study confirmed these findings. There was no significant difference in the rate of composite events between 12 months and 24 months DAPT at 48-month follow-up (3.2% versus 3.8%, p=0.26), but there was a significant increase in frequency of major bleeding with 24 months DAPT (p=0.026).26 The ARCTIC-Interruption study reported that major bleeding was more common with extended DAPT, but there was a non-significant difference in the occurrence of the composite of death, STH, stroke, or urgent TVR.27
The DAPT study was a trial recruiting the largest patient populations and therefore may be more generalisable in clinical practice. The study aimed to determine the benefits and risks of 30 months versus 12 months DAPT in >20,000 patients receiving stent implantation. Unlike previous trials, the therapy and allocation were blinded with both first and second-generation DES as well as clopidogrel and prasugrel. It showed a significant reduction in STH (hazard ratio [HR]: 0.29; 95% confidence interval [CI]: 0.17–0.48) and MI (HR: 0.47; 95% CI: 0.37–0.61) but with a concurrent increase in the risk of major bleeding (HR: 1.61; 95% CI: 1.21–2.16) and non-cardiovascular mortality (HR: 2.23; 95% CI: 1.32–3.78) with extended DAPT. However, the exclusion of those patients at high risk of ischaemic events and bleeding in the first 12 months in the DAPT study may not represent real-world patients and so the generalisability is limited.28
The OPTIDUAL study compared patients receiving 48 months and 12 months DAPT. This study failed to demonstrate the superiority of extended DAPT in reducing all-cause mortality, MI, stroke, or major bleeding compared to 12 months DAPT after DES implantation (p=0.17). This study showed a non-significant difference in all-cause mortality as well as non-cardiovascular mortality. However, the study was limited due to early termination and a low sample size that may not have the statistical power to demonstrate the beneficial effect of extended DAPT. Excluding patients with malignancies or other coexisting conditions associated with a life expectancy of <2 years might have excluded a high-risk group.29 There is a need for more studies to be conducted on patients without malignancies or other coexisting conditions associated with a life expectancy of <2 years.
Meta-Analyses
Meta-analyses of the above-mentioned trials17-21,23,24,26-29 demonstrated the beneficial effect of extended DAPT beyond 12 months on reducing frequency of STH and MI. However, there was a significant increase in the frequency of major bleeds and all-cause mortality as compared with 12 months DAPT.11,30 Extended DAPT was especially protective in reducing the risk of recurrent MI for patients with prior MI at high risk of late ischaemic events than early discontinuation of DAPT before 12 months (risk ratio: 0.70; 95% CI: 0.55–0.88, p=0.003).31 Extended DAPT may also protect from the occurrence of atherothrombotic events outside the stented segments throughout the coronary vasculature.32 The increase in all-cause mortality was driven by non-cardiovascular mortality,10-12 although no significant association was found between extended DAPT and non-cardiovascular mortality in a recent study evaluating the effect of extended DAPT on mortality.33 The inclusion criteria for this analysis, which included the trials comparing DAPT ≥6 months versus 0–6 months DAPT regardless of stent types implanted, may be responsible for this contradiction. One meta-analysis found no difference regarding the risk of all-cause and cardiac mortality. The rate of STH in short-term DAPT group could also be diminished by using safer and more effective second-generation DES.34 On the other hand, all the meta-analyses indicated that shorter duration did not differ from 12 months DAPT with respect to efficacy or safety endpoints and actually could be protective in major bleeding.
These meta-analyses were limited by the heterogeneity in protocol designs, the inclusion and exclusion criteria of populations, the definitions of clinical events, and the lengths of follow-up. Patients and interventions in the included studies might not be representative of real clinical settings. These analyses did not include the OPTIDUAL study. The overall effect on short-term versus extended DAPT should therefore be cautiously interpreted and updated when new evidence becomes available.
FACTORS ASSOCIATED WITH DUAL ANTIPLATELET THERAPY DURATION
Diabetes Mellitus
Diabetes mellitus is a risk factor for thrombotic events after stent implantation. There was some evidence demonstrating a relationship between diabetes and DAPT duration, but it was not sufficient to suggest an optimal duration of DAPT in patients with diabetes. A study suggested that prolonged DAPT duration was associated with a decreased risk of death and MI in diabetic patients.35 This finding was further confirmed by the subgroup analysis of the EXCELLENT trial, which found greater benefits from 12 months than 6 months DAPT in diabetic patients.19 In addition, this evidence was confirmed in recent reports concerning the advantage of newer-generation DES in diabetic patients.36,37 However, these findings were not confirmed by a recent sub-study from the SECURITY trial, in which no benefit of extending DAPT beyond 6 months in the prevention of ischaemic or bleeding events was observed in diabetic patients.38 The effect of stent type on diabetic patients will accordingly affect the decision on DAPT duration39 and should therefore be examined in randomised controlled trials with a sufficient number of patients.
High Bleeding Risk
Extended duration of DAPT could prevent late and very late STH but at the price of increased bleeding risk. Current guidelines recommend shorter durations of DAPT with second-generation DES in patients with higher bleeding risk (e.g. advanced age, renal insufficiency, history of transient ischaemic attack, stroke).6,7,40 One month’s DAPT following BMS implantation has also been given for patients at a high bleeding risk, but this works for the BioFreedomTM (Biosensors Europe, Morges, Switzerland) DES and not BMS, as shown in the LEADERS FREE trial.41 It is important to predict bleeding complications as well as thrombotic events. Predicted models have been developed for bleeding events.42 However, the availability of emergency medical care, as well as clinical presentation of patients, may influence the complications of major bleeding, while clinical predictors such as ACS, diabetes, and renal insufficiency were not only reported as risk factors of STH but also of bleeding, which might make it difficult to balance thrombotic prevention and bleeding risk. More data balancing STH and bleeding risks in varied clinical settings are highly necessary.
UNRESOLVED ISSUES AND CLINICAL IMPLICATIONS
The results of current meta-analyses indicate that recommendations of DAPT duration after DES implantation should be carefully individualised by weighing up benefits and risks. The standard 12 months DAPT is a reasonable trade-off justified by the totality of the current evidence from randomised controlled trials. However, the risks of thrombosis and bleeding are different in every patient.
Newer Generation Drug Eluting Stents
An extended duration of DAPT shows advantages in preventing late and very late STH in patients with first-generation DES implantation. The adverse consequence of DAPT discontinuation on increased STH has led to the development of a newer generation of DES.43 A significant decrease in STH with second-generation DES was observed when DAPT was ceased before 12 months, but no differences were observed between first and second-generation DES with prolonged DAPT.44 New-generation DES have been suggested to be safer than both BMS and early-generation DES with a significantly lower risk of early, late, and very late STH in short and long-term follow-up.45 With a more favourable healing profile and improved re-endothelialisation properties, a shorter duration of DAPT may be considered as a safe, effective, or beneficial strategy for patients undergoing PCI with newer-generation DES and modern interventional techniques, especially in those at high bleeding risk. However, there were few stent-specific studies performed. A recently published randomised sub-study of the I-LOVE-IT 2 trial has shown the non-inferiority in safety and efficacy of 6–12 month DAPT after the implantation of novel DES (biodegradable polymer sirolimus-eluting stents).46 More DES-specific studies are required, although it is a challenge to conduct randomised controlled trials on newer-generation DES because of the lower rate of STH events.
Newer P2Y12 Inhibitors
Newer P2Y12 antagonists with greater suppression of platelet activity, and hence lower rates of recurrent ischaemic events, are increasingly used instead of clopidogrel.47 However, evidence on the optimal duration of DAPT with these newer inhibitors is limited. Prasugrel and ticagrelor are recommended on equal terms with clopidogrel in patients with ACS or DES implantation in current guidelines.6 In fact, newer P2Y12 antagonists may result in a significant difference in the balance between ischaemic and bleeding risk. Prasugrel and ticagrelor were found to be more effective than standard or even high-dose clopidogrel in STEMI patients after implantation of either DES or BMS.48 Further randomised controlled trials are needed to examine the effect of novel P2Y12 inhibitors under diverse durations of DAPT.
Clinical Choice
The clinical decision on DAPT duration should be individualised according to each patient’s ischaemic and bleeding profile. The protection of extended DAPT duration on STH is counterbalanced by an increased risk of bleeding and all-cause mortality.
The application of a prolonged DAPT regimen for >12 months would be reasonable in selected patient populations beyond prevention of STH. It could be contemplated for prevention of MI in patients having a high ischaemic risk but a low bleeding threat and with a good tolerance of the initially recommended DAPT duration. Patients with a history of clinically significant intracranial or gastrointestinal bleeding should be allocated short-term DAPT. Premature discontinuation of DAPT should be avoided and newer-generation DES should be applied to prevent late and very late STH events. The DAPT Score, developed from the DAPT trial, could be useful to predict the risk of bleeding and decide whether to continue DAPT beyond 12 months or not. Several risk scoring systems have been developed to predict future ischaemic and bleeding risks after PCI and identify patients who can benefit from different DAPT durations. Patients with a DAPT Score ≥2 may consider extending DAPT >12 months regardless of MI status and vice versa.49 Patients with a PARIS Score at 0–3, 4–7, and ≥8 may have low, intermediate, and high bleeding risk, respectively. Shorter DAPT durations should be considered if a higher PARIS Score is predicted.50 However, the predictive accuracy of these scores should be further verified in large studies with more diverse patients before we can use them to guide clinical decision-making on duration and potency of DAPT.
Future Studies
Before implementing the optimal choice of DAPT for DES receivers, the limitations of clinical trial evidence must not be overlooked. The major limitation of current meta-analyses is a lack of patient-level data, which precludes covariate-adjusted and time-to-event analysis of studies of different durations of DAPT. It is therefore necessary to conduct better-powered, larger, long-term follow-up clinical trials to shed more light on deciding the optimal duration of DAPT. Subgroup analysis can then be conducted to provide some evidence on risks and benefits in specific groups of patients. The potential effects of genetic variations on platelet responsiveness may influence the effectiveness of DAPT51 and should therefore be added to further studies.
When new stents and antiplatelet drugs are introduced, optimal DAPT duration needs to be re-evaluated. The clinical presentations and bleeding and ischaemic risk profiles of patients are important factors to be taken into consideration as well. Any change in the clinical profile of patients may alter the risk-benefit balance or compliance with DAPT. Potential risk factors of STH and bleeding are summarised in Table 3. Therefore, physicians must carefully weigh up the potential benefit with respect to a reduced risk of ischaemic events and late STH against an increased risk of bleeding before making the therapeutic decision.
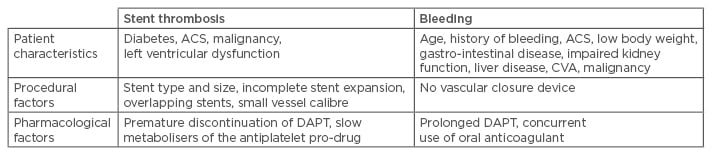
Table 3: Risk factors for stent thrombosis and bleeding after percutaneous coronary intervention.52
ACS: acute coronary syndrome; CVA: cerebrovascular accident; DES: drug-eluting stent; DAPT: dual
antiplatelet therapy.
CONCLUSIONS
DAPT prevents STH in patients after DES implantation but there are bleeding complications. As the balance of risk and benefit differs among patients, physicians should balance the risk of bleeding against benefits of preventing ischaemic events in each patient carefully and explain to patients. Large randomised controlled trials with newer generations of DES and P2Y12 antagonists should be conducted to provide evidence with sufficient statistical power.








