Abstract
Transcatheter aortic valve implantation (TAVI) is an alternative, less invasive method to use for aortic valve replacement in high-risk patients. This operation allows a faster recovery, reduced tissue damage, less postoperative pain, increased patient satisfaction, reduced intensive care unit (ICU) stay, avoidance of ICU admission, reduced hospital stay, and reduced wound infection rates. A retrograde transfemoral approach is commonly used in TAVI procedures. The role of the anaesthetist is important for a successful outcome. General or local anaesthesia, with or without conscious sedation, may be used according to patient characteristics, the presence of comorbidities, and the preference of the surgical team. There is no general consensus regarding which patients should receive general or local anaesthesia during TAVI operations; therefore, the surgical team’s preference has an important influence on the selection of anaesthetic technique. There are many studies in the literature relating to the anaesthesia technique used in TAVI operations. No matter which technique is used, anaesthetists should provide and maintain optimal haemodynamic stability during the procedure. On the other hand, anaesthetists should be cautious of possible procedural complications, such as hypotension, ventricular fibrillation, permanent pacemaker requirement, and emergency aortic valve replacement requirement.
INTRODUCTION
Aortic stenosis is an acquired degenerative valvular disease and is frequently seen in the elderly population, who commonly have medical comorbidities such as severe left ventricular dysfunction and renal and respiratory diseases. Patients diagnosed with aortic stenosis have a high mortality rate (4–18%) for open-heart surgery.1 Transcatheter aortic valve implantation (TAVI) is an alternative, less invasive method for high-risk patients, compared with surgical aortic valve replacement (SAVR).2 This procedure results in a faster recovery, reduced tissue damage, less postoperative pain, increased patient satisfaction, reduced intensive care unit (ICU) stay, avoidance of ICU admission, reduced hospital stay, reduced wound infection, reduced sternal dehiscence, avoidance of resternotomy, no activation of coagulation cascade, reduced bleeding, reduced release of vasoactive substances, reduced myocardial dysfunction after cross clamp, and a reduced use of resources. On the other hand, TAVI may cause serious complications, including haemodynamic instability requiring inotropic support, embolisation of aortic material, aortic regurgitation, complete heart block requiring a permanent pacemaker, vascular access damage and haemorrhage, or the metal frame stent may be placed incorrectly.3 A co-ordinated multidisciplinary approach, including a cardiologist, cardiothoracic surgeon, anaesthetist, perfusionist, and cautious anaesthetic management are essential for the success of TAVI.4
THE IMPORTANT ROLE OF THE ANAESTHETIST IN TRANSCATHETER AORTIC VALVE IMPLANTATION
Anaesthetists who provide support for TAVI have critical responsibilities before, during, and after the procedure. The anaesthetist should be knowledgeable of cardiothoracic anaesthesia, fluoroscopy, and echocardiography. Primarily, good preoperative risk evaluation should be provided by the anaesthetist to reduce the morbidity and mortality risk associated with TAVI. Anaesthetists should identify risk factors such as previous interventional procedures, signs of congestive heart failure, and laboratory evaluations, and discuss these risk factors with the procedure team and the patient beforehand.5 They should develop strategies to make the procedure as safe as possible and should also communicate clearly with the procedure team. On the other hand, the anaesthetist should provide haemodynamic stability and stay alert for complications, such as severe haemodynamic instability, haemorrhage/hypovolaemia, major vascular rupture, left ventricle perforation, hypothermia, conduction block/arrhythmias, cerebrovascular accidents, incorrect placement of the valve/paravalvular regurgitation, coronary ostial occlusion, or embolisation, that may occur during TAVI.6 After the procedure, the anaesthetist should be careful to stay aware of complications such as bleeding from the cannulation site and arrhythmias.5,6
ANAESTHESIA TECHNIQUES
Transfemoral, subclavian, axillary, direct aortic, apical, and transcaval approaches have all been described for the TAVI procedure.2,7 The retrograde transfemoral approach is the most commonly used.2 The role of the anaesthetist is important for a successful outcome because elderly patients often have multiple comorbidities and organ dysfunction.1 Inadequate haemodynamic management during the TAVI procedure may lead to morbidity and mortality and the postoperative period may be complicated. Therefore, optimal haemodynamic stability should be maintained throughout.3
General anaesthesia (GA) or local anaesthesia (LA) with conscious sedation (LACS) may be used according to patient characteristics, the presence of comorbidities, and the preference of the surgical team.4 GA was preferred in the initial learning process in clinics and later preferred in patients with coexisting diseases such as heart failure, obesity, pulmonary disease, and cardiogenic shock.8,9 Covello et al.8 analysed 69 patients (42 patients received LACS and 27 received GA) who underwent TAVI. They concluded that GA or LACS were both valid alternative techniques that can be titrated according to patient characteristics. Bergmann et al.9 performed TAVI under sedation in 100 patients. Sedation alone was required in 83 patients; in 17 patients sedation had to be converted to GA due to interventional complications. There were no significant differences between LACS and GA groups in terms of procedural or postoperative results (length of ICU stay, 30-day and 1-year mortality). They concluded that TAVI can safely be facilitated by sedation in the majority of patients. Oguri et al.10 compared the clinical outcomes in patients who underwent transfemoral-TAVI under GA or LACS. They reported no significant differences in terms of Valve Academic Research Consortium (VARC)-defined complications (myocardial infarction, stroke, and vascular and bleeding complications), other procedural complications, procedure success, or cumulative 30-day and 1-year survival rates. On the other hand, they found more common post-procedural aortic regurgitation ≥mild in the LACS group, compared with the GA group. They thought that transoesophageal echocardiography (TOE) support during TAVI might reduce the incidence of post-procedural aortic regurgitation. Indeed, Berry et al.11 have reported that TOE provides key anatomical and functional information during TAVI procedures. In the period prior to the procedure TOE is used to assess aortic valves, to measure aortic root diameters and left ventricular outflow tract dimensions, to evaluate left ventricular structures and mitral valve functions, and to evaluate thoracic aortas anatomically. During the procedure, TOE provides visualisation of the prosthetic valve position, the effects of the balloon valvuloplasty, and rapid diagnosis of complications (such as pericardial effusion and iatrogenic mitral regurgitation). In the period after the procedure, prosthetic valve assessment, measurement of aortic root diameters and left ventricular outflow tract dimensions, and evaluations of left ventricular structure, mitral valvefunction, and thoracic aorta are performed via TOE.11
In a recent study,12 we presented short-term results of the first TAVI applications used in our institute. An Edwards SAPIEN valve was implanted, followed by a balloon aortic valvuloplasty via transfemoral approach for all patients. All procedures were performed under GA accompanied by TOE. There were no mortality or serious complications during the procedures and the success rate was 100% at our institute. In addition, we reported a low mortality rate in the first 30 days (4%) and 6 months (16%). Gümüş et al.13 also performed all approaches under GA with fluoroscopic and TOE guidance reported a 7% mortality rate in the first 30 days. However, Dehédin et al.14 applied transfemoral TAVI using GA or LACS. They reported lower intraoperative catecholamine requirements and volume expansion, shorter procedure durations, and shorter hospital stays in the LACS group, compared with the GA group. They found similar periprocedural outcomes, 30-day mortality rates, and lengths of stay in the ICU in both groups.
There is no consensus regarding which patients should receive GA or LA during TAVI operations. Therefore, the surgical team’s preference has an important effect on the selection of anaesthetic technique. GA requires tracheal intubation and mechanical ventilation, which leads to respiratory compromise, delayed extubation, haemodynamic instability, prolonged ICU stays, and haemodynamic instability. However, the GA technique is preferred by surgical teams in the initial learning process because it facilitates the management of procedural complications due to the patient’s immobility and allows the use of TOE. On the other hand, LA provides simple neurological monitoring, improved patient satisfaction, and reduced morbidity. However, LA catheter placement may cause patient discomfort, and possible patient movement may increase the risk of prosthesis misplacement.4 Vavuranakis et al.15 published initial experiences in a total of 30 patients treated with TAVI using only LACS. They reported that TAVI, without GA, using a CoreValve® prosthesis, is a safe procedure. In another study,16 a similar procedural success rate, 30-day mortality, and 30-day combined safety were reported in patients undergoing TAVI with GA or LACS. In addition, the LACS group had a shorter procedural time, ICU stay, and hospital stay, unlike the GA group. Ben-Dor et al.17 also demonstrated the feasibility and safety of performing TAVI guided by TOE without the necessity of GA. In a similar study, Motloch et al.18 found significantly less periprocedural adrenergic support, shorter intervention times, and lower labour costs in patients undergoing TAVI under LACS compared to the patients under GA. Attizzani et al.19 reported shorter post-procedural hospital stays, lower costs, and similar safety profiles in patients who received LACS compared to patients receiving conventional strategies.
In a recent study, Piayda et al.20 evaluated the safety and feasibility of TAVI via femoral access, under LA only (without concomitant sedation) in a total of 215 patients. Of these patients, 40 (18.6%) received additional sedation during the procedure due to inadequate pain control or agitation, and conversion to GA was applied in 7 patients (3.3%). They reported a significantly longer duration of ICU stay in the group requiring additional sedation, compared with patients who only received LA or GA. They suggest that TAVI with LA alone may be considered as the primary option in many patients. Goren et al.21 assessed the feasibility and safety of TAVI under sedation in an observational study. They reported significantly less catecholamine and intravenous fluid requirements, a shorter total procedural time, and less post-procedural pulmonary complications in the sedation group. In another study, Petronio et al.22 assessed the safety and non-inferiority of LA versus GA in a large cohort of patients undergoing TAVI. They observed a shorter procedural time, lesser use of a surgical vascular access, a lower incidence of acute kidney injury Stage 3, a lower rate of bleeding and major vascular access-site complications, and a shorter length of hospital stay in the LA group. They concluded that TAVI under LA is as effective and safe as TAVI under GA in experienced centres. Greif et al.23 achieved good clinical outcomes with TAVI under LA performed with only mild analgesic medication under fluoroscopic guidance. The Sentinel European TAVI Pilot Registry analysed 2,807 patients from ten participating countries treated transfemorally with either LA (1,095 patients, 39%) or GA (1,712 patients, 61%). They found an increase in LA use over time, and contrary to other results similar death and survival rates for the two approaches in the first year of the study. In another report, Mayr et al.25 researched the effect of sedation and GA on cerebral oxygen saturation and neurocognitive outcomes in patients undergoing TAVI. In this randomised controlled trial, they reported similar cumulative cerebral desaturation and neurocognitive function levels between groups. However, they observed a higher incidence of adverse events (bradypnea: the need for airway maneuvers and bag-mask ventilation) in the sedation group. In a meta-analysis, Fröhlich et al.26 compared LA with monitored anaesthesia care (MAC) versus GA in patients undergoing transfemoral TAVI. MAC was defined as cardiovascular and respiratory monitoring of the patient by a qualified anaesthetist, who may or may not be administering concomitant sedation. They reported similar mortality and safety endpoints in both and that patients with GA were more likely to need catecholamine support. Also, they observed a shorter procedural times and in-hospital stays in patients undergoing TAVI with MAC, compared with GA.
CONCLUSION
TAVI continues to be an alternative to SAVR for high-risk patients. There are many studies in the literature related to the anaesthesia technique used in TAVI operations (Table 1). Based on the results of these studies, LA with only mild analgesic medication, LACS, or GA may be used successfully in selected cases undergoing TAVI. The use of LA techniques in TAVI operations has been steadily increasing over past years, and has been associated with improved clinical outcomes. In the selection of the most appropriate anaesthetic technique for a patient, the first step is an attentive preoperative assessment with the anaesthetist evaluating patient comorbidities and haemodynamic status. The procedure team’s experience is another factor for the selection of an appropriate anaesthesia technique. No matter what technique is used, the anaesthetist should provide and maintain optimal haemodynamic stability during the procedure. On the other hand, the anaesthetist should be cautious for possible procedural complications such as hypotension, ventricular fibrillation, permanent pacemaker requirement, and emergency SAVR requirement. If LACS is used, the anaesthetist must be ready to perform full GA at any moment during the procedure. Further studies are needed to determine whether the anaesthesia technique used may improve short and long-term clinical outcomes of TAVI.
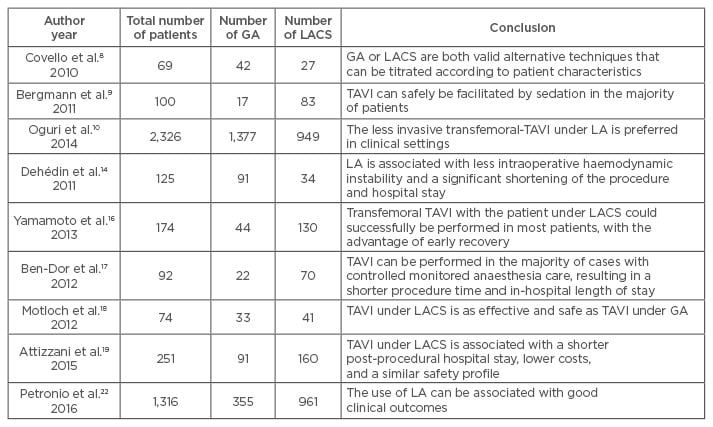
Table 1: Mentioned studies comparing local anaesthesia with conscious sedation versus general anaesthesia in patients undergoing transcatheter aortic valve implantation.
TAVI: transcatheter aortic valve implantation; GA: general anaesthesia; LACS: local anaesthesia with conscious sedation; LA: local anaesthesia.








