Abstract
With nano-based products becoming ubiquitous across all therapeutic areas, especially the area of skin care, it has become imperative to review the correlation between the unmet needs and the pipelines of available products. Atopic dermatitis (AD) is prevalent across different regions of the world with an incidence rate varying from 15–30% in children and adults. The pathogenesis of AD is a complex interplay between defects in skin barrier function, environmental and infectious agents, and immune abnormalities. Furthermore, although the role of reactive oxygen species has been studied in AD and other skin diseases to some extent, its importance in AD has rarely been investigated. The limitations associated with the use of currently available therapies like topical corticosteroids (first-line) and/or topical calcineurin inhibitors, and the use of other over-the-counter products to manage the sleep disturbances and skin infections, create a need for other innovative solutions. Nano-intervention forms a large panel of delivery aids, including lipidic and polymeric nanoparticles, liposomes, silica nanoparticles, hydrogels, and several other delivery systems. These interventions are mainly designed to achieve higher drug encapsulation, greater stability, and higher skin permeation. This review aims to give an overview of the epidemiology of AD, the pathogenic events, and the challenges present with currently available therapies. There is a special focus on the recent developments in various nanocarrier technologies for treating AD.
INTRODUCTION
Atopic dermatitis (AD), a multifaceted, chronic relapsing inflammatory skin disease, is commonly associated with other atopic manifestations, such as food allergy, allergic rhinitis, and asthma, and affects both children and adults with prevalence rates varying from 15–30%.1 The phrase AD is a portmanteau of atopy, or atopic syndrome, and dermatitis. The term ‘atopy’, which was coined by Coca and Cooke in 1923, is used for any IgE-mediated reaction (even those that are appropriate and proportional to the antigen); however, it may involve a genetic component, though contact with the allergen must occur before the development of hypersensitivity reactions.2,3 Atopy can be present in the form of asymptomatic sensitisation of one or more of the atopic diseases, such as AD, fever, and asthma. Dermatitis, on the other hand, is a medical condition associated with reddening, swelling, and soreness of the skin, with small blisters resulting from direct irritation of the skin by an external agent or an allergic reaction.4 Over the years, other names have been proposed for the disease, for instance, Prurigo Besnier (Besnier’s itch), named after the French dermatologist Ernest Besnier (1831–1909).
The term ‘allergic march’ (also called ‘atopic march’) refers to the natural history of atopic manifestations, characterised by a typical sequence of events that include IgE antibody responses and then the appearance of clinical symptoms early in life that persist over years or decades and often remit spontaneously with age.5
The underlying cause of AD is a defective skin barrier that results in dry, itchy skin, which is aggravated by mechanical injury inflicted by scratching. The interplay of the innate and adaptive immune systems in the pathogenesis of AD has been evidenced.6
In recent years, there has been increasing evidence regarding the role of oxidative stress (OS) in AD. It is also well known that OS promotes tissue inflammation through upregulation of genes that code for proinflammatory cytokines. Inflammatory cells in turn release free radicals when activated. Given its prominent inflammatory component, it is conceivable that OS may play a role in the pathogenesis of AD.7 The current review attempts to detail the epidemiology, the pathogenic events related to AD, the challenges in the present drug therapy, and the scope of nano-based drug delivery systems for AD.
EPIDEMIOLOGY
The prevalence of AD is estimated to be 15–30% in children and adults, and the incidence has increased 2–3-fold during the past decades in industrialised countries.1 The World Health Organization’s (WHO) 2010 Global Burden of Disease survey ranked AD first among common skin diseases with respect to disability-adjusted life-years and years lived with a disease.8 An international study of 1 million subjects was carried out to assess the epidemiology and geographic variability in the prevalence of AD, which was conducted in three phases.8 Both the prevalence and the region of greatest AD prevalence continues to vary around of the world. Nigeria, the UK, and New Zealand were previously the areas with the highest prevalence; however, now Latin America has emerged as a region of relatively high prevalence in follow-up data.9 Many developing countries have also seen a marked increase in atopic disease occurrence.
The incidence of AD has been increasing in India over the last four decades. AD was the most common dermatosis in children registered to a paediatric dermatology clinic, where it constituted 28.46% of all registered patients. A study report from the Postgraduate Institute of Medical Education and Research, Chandigarh, India, revealed the prevalence of AD in 210 infants (up to 1 year) and children (n=462) in different seasons.10
PATHOGENESIS
The pathogenesis of AD has puzzled researchers for decades. Although important milestones have been achieved in explaining the mechanisms of precipitation of AD in genetically predisposed individuals, there are still many omitted points that need to be discovered in order to put forth a unifying concept.11 The occurrence of recurrent itch and dermo-epidermal inflammation is manifested histologically as hypertrophy of the dermis and epidermis with the presence of eosinophils, macrophages, and T cells.12 Furthermore, the inflammatory response in AD begins when an IgE-associated Langerhans cell in the skin binds an antigen and presents the antigen to T cells, which leads to the release of numerous cytokines, including various IL. These mediators lead to the recruitment of more inflammatory cells, resulting in eczema.13 Overall, the development of AD is considered to be multifactorial, with complex interactions between susceptibility genes, immaturity and/or abnormalities in barrier function, and environmental factors (Figure 1).14
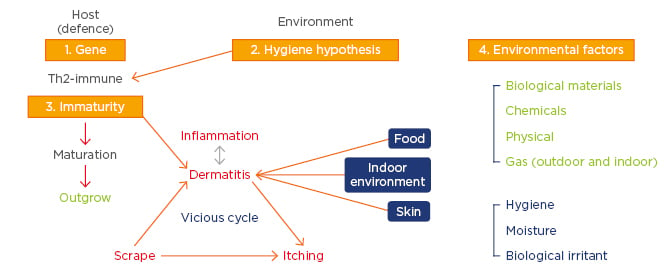
Figure 1: Multifactorial pathogenesis of atopic dermatitis.
There is a complex interplay between defects in skin barrier function, inflammation, immunity, and OS. As previously mentioned, the role of reactive oxygen species remains an unexplored area by researchers, although they are believed to play a role in AD.15 OS refers to an imbalance that occurs between the generation of free radicals and the available antioxidant defence system in the body. Its implication in AD has been reported in the literature for the last 15 years.16 Dermal inflammation, which is the characteristic feature of AD, is aggravated by OS. The role of OS in the activation of NFκB pathways, which in turn activates the gene expression and finally the synthesis of antioxidant enzymes, has been reported.16 However, NFκB pathway activation also induces expression of proinflammatory cytokines (IL-6, IL-8, IL-9, and IL-33), which, in turn, enhances dermal inflammatory infiltrate and causes the release of histamines in the skin to further worsen the symptoms.17
Several animal studies have reported the involvement of OS in causing itching and scratching, even in nonatopic animals. Repeated application of chemicals, such as formaldehyde, or the use of intradermal hydrogen peroxide may provoke itching through increased expression of IL-4 or by the histamine-independent pathway, respectively.18
OS damages the epidermal keratinocytes by disrupting the DNA, the cellular enzymes, and/or the cell membrane structures through lipid oxidation. These intracellular changes are manifested histomorphologically in the form of epidermal oedema or spongiosis and a disrupted stratum corneum. Ceramides are one of the most important lipids involved in maintaining an intact skin barrier. They are composed of sphingosine and fatty acids, which are produced during the process of keratinisation in the stratum corneum. The presence of an intact epidermal barrier helps limit the entry of allergens and other infectious agents and, thus, prevents the loss of transdermal water. Numerous studies have reported that the skin barrier is directly damaged by OS initiated by external pollutants.19
Environmental pollutants, such as cigarette smoke, bind to aryl hydrocarbon receptors and induce reactive oxygen species production, DNA damage, and inflammatory cytokine production to cause skin inflammation. In contrast, certain flavonoids bind to aryl hydrocarbon receptors, resulting in the activation of nuclear factor-erythroid 2-related factor-2 (Nrf2) to produce key molecules that protect cells from oxidative damage.20 Another source of OS might be skin microbes. A close association of four main factors in the aetiology and pathogenesis of AD has been suggested; these are genetic predisposition, impaired immunity, epidermal barrier dysfunction, and environmental factors.21
It was demonstrated that urine markers of OS are altered in children with AD, including 8-hydroxydeoxyguanosine (8-OHdG), nitrite or nitrate, and selenium.22 Those marker levels are higher in children with AD than in non-AD children. It was suggested that impaired homeostasis of oxygen/nitrogen radicals and increased OS are involved in the pathophysiology of childhood AD. Chung et al.23 also found that blood antioxidant capacity was significantly lower and serum malondialdehyde was higher in preschool children with AD compared to controls. More recently, Amin et al.24 conducted a case-control study on eczema patients with healthy individuals as controls. They found that, compared to the control group, patients with eczema had a significantly higher level of lipid peroxidation, which was determined by measuring serum malondialdehyde, and lower levels of antioxidants, including vitamins A, C, and E. Similar findings of the presence of OS and increased lipid peroxidation were reported in patients with alopecia areata, an inflammatory skin condition closely related to AD.25 Subsequently, Tsukahara et al.26 and Naziroglu et al.27 observed OS and altered antioxidant defences in children with acute onset of AD. They found that urinary glycosylation end products and bilirubin oxidative metabolites were significantly higher in AD children during hospitalisation.
Genetic Factors
Food allergens may be the major trigger of AD in early life, after which the role of environmental aeroallergens becomes more important and may be associated with respiratory sensitisation. The mode of inheritance and genes involved are not clear.28
Filaggrin, an important protein that binds to keratins associated with keratinocyte differentiation, is important in maintaining the integrity of the skin barrier. There is decreased production of filaggrin due to genetic defects leading to skin barrier dysfunction and transepidermal water loss, which causes eczema. This leads to increased penetration of allergens into the skin, resulting in allergic sensitisation, asthma, and hayfever.29
Skin Barrier Dysfunction
The epidermis functions as a primary defence and acts as a biosensor to the external environment. Skin barrier defects promote easy entry for pathogens, allergens, and other environmental insults (toxins, irritants, and pollutants) and are now considered a primary mechanism for the development of AD.30 The skin barrier function is impaired in AD as a result of multiple abnormalities that are responsible for the barrier defect, including reduced lipids (i.e., ceramide and sphingosine). Clinically, the disrupted skin barrier function of atopic skin leads to increased transepidermal water loss and increased penetration of irritants, allergens, and microbes.31
Immunological Responses
The immune response observed with AD is characterised by biphasic inflammation. A Th2-based immune response (IL-4, IL-13, thymic stromal lymphopoietin, and eosinophils) is predominant in the initial and acute phase of AD, while in chronic AD skin lesions, a Th1/Th0 dominance has been described (IFN-γ, IL-12, IL-5, and granulocyte-macrophage colony-stimulating factor).32
Cytokines and chemokines are key factors in the progression of AD. Leukocytes, and especially monocytes, from atopic patients demonstrate elevated phosphodiesterase activity, leading to decreased levels of cAMP and increased production of prostaglandin and IL-10, which inhibit Th1 function and enhance IgE production.33
Host and Environmental Factors
The presence of food sensitisation and allergy early in life has been reported in cases of severe AD. Around 50–70% of children with an early onset of AD are sensitised to one or more allergens, with cow’s milk, hen’s eggs, and peanuts the foods most frequently involved, but also house dust mite, pollen, and pets. Food allergy is common in children with AD, with an association that has been proposed to range from 20–80%, although the more commonly accepted figure is 30%.34
THERAPEUTIC APPROACHES AND MANAGEMENT OF ATOPIC DERMATITIS
AD is still not curable, with many patients experiencing a chronic phase of the disease. Accordingly, the treatment of AD aims to:
- Minimise the number of exacerbations of the disease, so-called ‘flares’.
- Reduce the duration and degree of the flare, if flare occurs.
The first aim relates to prevention while the second relates to treatment. Prevention can be attained at three stages, which include primary, secondary, and tertiary care programmes.
Primary and Secondary Prevention
The proposal of food evasion in the prevention of AD is based on the perception that delayed exposure to antigens is a modifiable factor in AD. A direct causal relationship between food avoidance and prevention of AD is still unclear,35 but sensitisation to food allergens, such as cow’s milk and hen’s eggs, is associated with infantile AD.36 In established AD, the role of food is still contentious. Intervention involving the use of hydrolysed or amino acid-based formulas in children with significant disease has been reported.37
Tertiary Prevention
Prevention is best attained by trying to reduce the dryness of the skin, primarily via daily use of skin moisturising creams or emollients along with avoidance of specific and unspecific irritants, such as allergens and non-cotton clothing. As such, emollients have no direct effect on the course of the eczema. Furthermore, avoiding long, hot baths prevents skin dryness and application of an emollient is recommended to maintain a moist epidermis and augment the skin barrier function.
Currently Available Treatment Options
Topical Corticosteroids
Topical corticosteroids are the first-line anti-inflammatory treatment to control moderate-to-severe AD in both children and adults. Corticosteroids are hierarchically grouped into four different classes based on their vasoconstrictory abilities: mild, moderate, strong, and very strong preparations. Treatment with topical corticosteroids to reduce skin inflammation can also contribute to a reduction of skin colonisation with Staphylococcus aureus and may minimise a further trigger factor of AD.38
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCI) are compounds that represent a relatively safe and second-line class of topical anti-inflammatory, nonsteroidal therapy that work by inhibiting calcineurin, thereby preventing the dephosphorylation activity of phosphatase and the production and release of inflammatory cytokines and T cell proliferation. TCI (e.g., pimecrolimus cream and tacrolimus [TAC] ointments) are not yet approved by the U.S. Food and Drug Administration (FDA) for children <2 years of age. In January 2006, the FDA issued a black box warning on these compounds regarding possible concerns about the potential of increased long-term malignancy risk owing to systemic immunosuppression and recommended that TCI not be used as first-line therapy.39
Phototherapy
Ultraviolet (UV) light, especially narrow-band UVB, is particularly suitable for treating adults with recalcitrant eczema. Narrowband therapy for the treatment of AD is believed to possess an equal or superior safety and efficacy compared to natural sunlight or UVB exposure. The use of a combination of broad-band UVA light and the photosensitising drug, psoralene, to treat severe recalcitrant eczema has also been reported.40
Antibiotics
S. aureus colonisation is a well-recognised complication in AD and a high rate of colonisation is directly correlated to its severity. As treatment with topical steroids alone can decrease the S. aureus colonisation,41 the use of systemic antibiotics is indicated for secondary bacterial infection only (primarily S. aureus). Community-acquired methicillin-resistant S. aureus (MRSA) has become an increasingly important therapeutic issue when treating abscesses but does not appear to be as prevalent in patients with AD who have secondary infections. However, this possibility is generally considered for unresponsive patients.41
Use of Anti-Allergics (Antihistamines and Leukotriene Antagonists)
First-generation antihistamines with sedative properties are useful as short-term adjuvants to topical treatment in cases of severe pruritus, while non-sedating antihistamines appear to have only a very modest influence on atopic eczema. The usefulness of leukotriene antagonists (montelukast and zafirlukast) for the treatment of asthma and allergic rhinitis has been reported, but they have shown limited success in severe AD.42
Systemic Immunosuppressant Treatment
Since oral corticosteroids are associated with serious side effects, a combination with a secondary immunosuppressant is generally a preferred mode of treatment. The latter include drugs (e.g., cyclosporine A, azathioprine, mycophenolate mofetil) and bioengineered immunomodulators (omalizumab, rituximab).43
Other Treatment Options
Complementary and alternative medicines have been used by 35-69% of patients with a dermatological disease. The treatments include natural health products, nutritional supplements, biomechanical therapies (e.g., massage therapies and mind–body therapies), and probiotics (non-pathogenic microbes).
INTERVENTION OF NANOTECHNOLOGY-BASED DRUG DELIVERY
Nano-intervention-based drug delivery is a rapidly growing field with potential applications in health and drug therapy. The efficacy of topically applied drugs in clinical dermatology is generally observed by their mechanism of action and their ability to pass through the protective skin barrier. Topical delivery of nanoparticles (NP) has been explored for attaining local and systemic therapeutic effects.
The skin provides a natural physical barrier against particle penetration, but there are opportunities to deliver therapeutic NP, especially in diseased skin and across hair follicle openings. While NP drug delivery has been touted as an enabling technology, its potential in treating local skin and systemic diseases has yet to be realised.
Several mechanisms elaborating the penetration and permeation of NP across the outermost rate-limiting barrier of the stratum corneum have been detailed and discussed. One hypothesis suggests the entrapment of the NP into the lipid matrix of the skin such that the drug can be slowly released into the dermal layers to achieve therapeutic efficacy.45 The release is affected by parametric evaluation of NP in terms of size, shape, charge, and surface properties, which eventually decide the degree of skin penetration. Furthermore, apart from assigning the role of the intrinsic parameters to affect the rate and extent of skin penetration, NP interaction with the physiological media also plays a significant role. Figure 2 discusses the absorption pathways to be considered for the evaluation of NP skin permeation.46
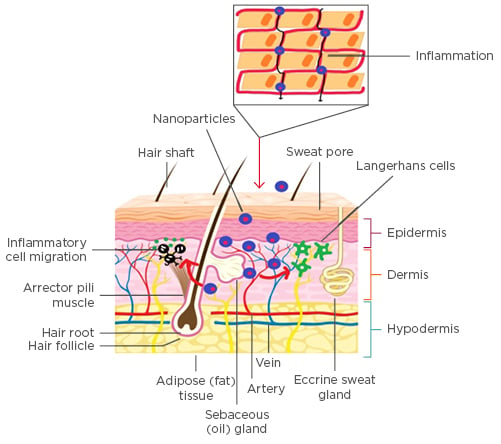
Figure 2: Nanoparticles permeation into the skin.
Nanotechnology can be used to modify drug permeation and penetration by controlling the release of active substances and increasing the period of permanence on the skin, besides ensuring a direct contact with the stratum corneum and skin appendages and protecting the drug against chemical or physical instability. Furthermore, the delivery of therapeutic agents without the need for chemical enhancers is desirable to maintain the normal skin barrier function. Treatment with chemical enhancers, such as surfactants and organic solvents, can cause not only a reduction in the barrier function of the skin but also irritation and damage to the skin.47
Generally, NP act as alleviating agents against hypersensitivity reactions and mechanisms. Most of the drug delivery particle technologies are based on lipid carriers; e.g., solid lipid NP and nanoemulsions (roughly 300 nm in diameter, which is now considered to be a microparticle), polymeric NP, liposomes, silica NP, and hydrogels that have been researched as alternative drug delivery options.48
Use of nanodrug delivery systems for enhancing the bioavailability of drugs and reducing the side effects of existing therapies should be explored to develop challenging products, and continued efforts to develop alternative treatments for the disease are underway. An alarming exponential rise in AD and other skin diseases is becoming a concern and has posed a challenge to researchers for identifying a suitable resolution. The literature has revealed vigorous efforts by scientists to explore the potential of antibiotics, corticosteroids, TCI, and even some natural polyphenolic compounds after suitable encapsulation into carrier systems for the treatment and resolution of AD. Various papers have been published based on nanotechnology-based topical drug delivery in recent decades; nonetheless, very little commercialisation is in the pipeline. Recently, a few studies based on the usefulness of nanosisation have been carried out and are discussed in detail below (Table 1).49-57
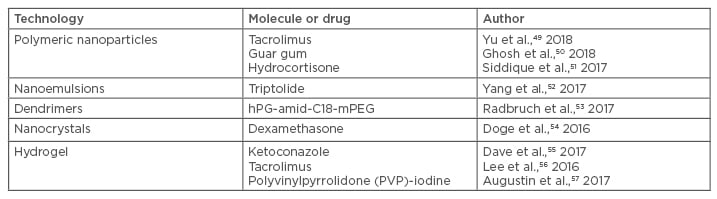
Table 1: List of nanocoutured drugs and their delivery system for atopic dermatitis.
Yu et al.49 developed a hybrid skin-targeting system encapsulating TAC (FK506) based on nicotinamide (NIC) and chitosan NP (CS-NP), i.e., FK506–NIC–CS–NP, to obtain the synergetic effects of percutaneous delivery in dermatitis. NIC was found to significantly increase the FK506 entrapment efficiency to 92.2% by preparation of CS–NP. It was found that the NIC–CS–NP system significantly enhanced FK506 permeation through and into the skin and deposited more FK506 into the skin in comparison with Protopic® (Leo Pharma, Ballerup, Denmark). The treatment efficacy on clinical symptoms, histological analysis, and molecular biology of the AD mice demonstrated that NIC–CS–NP with an approximately one-third dose of FK506 or Protopic was superior to that of Protopic alone, and the NIC–CS–NP vehicle exhibited the adjuvant therapeutic response and moderate anti-AD effects.49
Ghosh et al.50 studied the in vitro and in vivo efficacy of guar gum NP (GN) in AD. Balb/c mice ears were topically exposed to oxazolone (Oxa) to induce AD, and topically treated with GN. Application of GN showed a significant decrease in ear thickness on Day 28. The total cell count of skin cells decreased post topical application of GN on the affected skin. Further to a decreased eosinophil count, Th cell, and macrophage populations after application of GN were observed in histological studies. Moreover, the ear tissue showed a reduced cellular infiltration and epidermal thickness.50
Siddique et al.51 prepared the hydrocortisone (HC), topical glucocorticoid along with hydroxytyrosol (HT), and anti-microbial and antioxidant-loaded CSNP. Ten subjects were randomised to receive test (HC-HT CSNP) and vehicle samples (aqueous cream) and blood samples were analysed for blood haematology, blood biochemistry, and adrenal cortico-thyroid hormone levels. Skin biopsies were obtained to assess histopathological changes in the skin and were found to be stable. It was concluded that HC-HT CSNP (aqueous) cream is safe, well-tolerated, and non-toxic, which may be useful in treating AD.51
Yang et al.52 compared triptolide (TPL)-nanoemulsions and TPL-nanoemulsion gels and their effect on enhancing percutaneous permeation. Compared to TPL gels, significantly greater cumulative amounts of TPL-nanoemulsion gels and TPL nanoemulsions penetrated rat skin in vitro. In vivo microdialysis showed the concentration–time area under the curve for TPL-NP to be larger than the TPL-gels. The TPL-nanoemulsion gels had a significant treatment effect on dermatitis and eczema in the mice model and can reduce the expression of IFN-γ and IL-4. It was found that nanoemulsion gels could be promising percutaneous carriers for TPL and are expected to provide a new, low-toxicity, and are long-term preparation for the clinical treatment of dermatitis and eczema in transdermal drug delivery systems.52
Radbruch et al.53 reported on dendritic hPG- amid-C18-mPEG core-multishell nanocarriers (CMS) that represent a novel class of unimolecular micelles to facilitate topical therapy in skin diseases like AD. They tested the penetration behaviour and identified target structures of unloaded CMS after topical administration in healthy mice and in mice with Oxa-induced AD. CMS accumulated in the stratum corneum without penetration into deeper viable epidermal layers after having topical administration, indicating that barrier alterations in AD had no influence on the penetration of CMS in cases of Oxa-induced AD. Taken together, CMS accumulate in the stratum corneum in both healthy and inflammatory skin and appear to be highly biocompatible in the mouse even under conditions of AD; thus, CMS could potentially serve to create a depot for anti-inflammatory drugs in the skin.53
Doge et al.54 prepared nanocrystals of dexamethasone (Dex) formulated in ethyl cellulose nanocarriers and evaluated the formulation by microdialysis technique. The results indicated high efficacy in terms of molecular analysis and inflammatory markers.54
Dave et al.55 reported on liposomal gel containing ketoconazole and neem extract for the treatment of seborrhoeic dermatitis using the thin film hydration method. The anti-fungal activity of optimum liposomal formulation was carried out against Aspergillus niger and Candida tropicalis and inhibition zone measurements were 8.9 and 10.2 mm, respectively. The results indicated that developed liposomal gel of ketoconazole with neem extract could have great potential for seborrhoeic dermatitis and showed synergetic effect for the treatment.55
Lee et al.56 prepared the TAC-loaded topical hydrogel formulations composed of carbomer, carnosine, transcutol P (diethylene glycol monoethyl ether), and humectant for the treatment of AD. An in vitro drug release study revealed that the total amount of TAC released from hydrogels over 24 hours was approximately 30-times greater than that for the reference formulation and showed that there was higher skin permeation and retention of TAC (p<0.05), especially those with >10% of transcutol P. Furthermore, carbomer gel formulations with sufficient levels of transcutol P are good candidates for skin delivery of TAC and have potential as therapeutic agents for the treatment of AD.56
Augustin et al.57 reported liposomal polyvinylpyrrolidone (PVP)-iodine hydrogel, combining the antiseptic and anti-inflammatory actions of PVP-iodine with the drug delivery and moisturising properties of liposomes to treat infective dermatoses. In this prospective, single-arm, uncontrolled, open-label Phase II pilot study, patients with acne vulgaris (n=30), AD (n=20), impetigo contagiosa (n=10), and rosacea (n=10) received PVP-iodine (3%) hydrogel for ≤4 weeks. Global Clinical Severity score improved for all dermatoses (range: 0.5 for acne vulgaris [p<0.001] to 1.0 for impetigo contagiosa [p=0.011]). Improvements in pain, quality of life (Freiburg Life Quality Assessment), and Eczema Area Severity Index scores were also reported. The authors outlined a well tolerated treatment therapy, with burning (14%) or itching (9%) sensations as the most frequent adverse events. Thus, liposomal PVP-iodine hydrogel has potential use as an effective treatment for inflammatory skin conditions associated with bacterial colonisation.57
In addition to these aforementioned studies, efforts to incorporate drugs into NP,58 nanoemulsion,59 nanosuspension,60 nanofibrils,61 nanospheres,62 and liposomes63 have been discussed earlier and a detailed discussion of these has been given in a previous review on AD.64 Based on the previously discussed research findings, it can be affirmed that a nanotechnology-tailored product holds immense potential for the treatment and cure of AD.
CONCLUSION
Nanotechnology represents a key technology of the 21st century, offering excellent opportunities for both research and business. The therapeutic potential of nanotechnology-based products for AD is being realised at the laboratory level although many nano-cosmeceuticals have captured the market for their reflecting properties. It has provided a new avenue for the prevention and treatment of inflammation and its sequelae, with particularly promising advances for the alleviation of skin diseases.
Direct as well as indirect evidences substantiate reports on the usefulness of NP as carriers for topical administration, stimulating new and deeper investigations in the field. The success of nano-formulations relates to the drugs’ ability to deliver the active substance to the target organ at therapeutically effective concentrations, with minimal discomfort and side effects, thus increasing patient compliance.
As technology develops, there will be a movement towards a wider association between machines and nanotechnologies, with the probability of a deeper understanding of in vitro and in vivo perspectives for the mitigation of skin disorders. However, future studies are still warranted to establish its safety and efficacy in order to ensure consumer health.








