Meeting Summary
The symposium addressed the efficacy and safety of compounds currently available for treatment of hepatitis C virus (HCV) and chronic kidney disease (CKD) in North American and European countries, comparing data from trials and clinical practice. Prof Wedemeyer opened the meeting with a discussion of real-world experiences, with a focus on HCV genotypes (GTs) and resistance-associated variants (RAV). Prof Brown concentrated on trial and real-world data from patients with advanced liver disease, while Prof Craxí’s presentation focussed on chronic kidney conditions and infection. Prof Jacobson led the question and answer session and summarised the discussions.
Real-World Experience: What Have We Learned Overall?
Professor Heiner Wedemeyer
Although randomised controlled trials (RCTs) are considered the gold standard for evidence on clinical efficacy and safety, they cannot address all clinical questions and some essential data gaps can be obtained in the real-world setting. In contrast to RCTs, evaluation of real-world cohorts takes place in a clinical practice setting where patients are not randomised and data capture may or may not be protocol-driven. Data on patient baseline characteristics, treatment efficacy, along with patient safety and tolerability is either captured prospectively or analysed retrospectively. RCTs typically involve limited numbers of patients with extensive inclusion and exclusion criteria, while real-world cohorts present a larger, more diverse spectrum of patients with fewer restrictions. RCTs are typically shorter in duration and more expensive, whereas the clinical setting evaluates long-term efficacy and safety, and costs less per patient.1,2
There is a rich heritage of collecting data from the real world on anti-HCV treatments, and over the past 3 years many real-world studies of first and second-generation direct-acting antiviral (DAA) treatments have been published in different countries, answering key questions from Phase III trials. The European Association for the Study of the Liver (EASL) International Liver Congress (ILC) 2016 was of particular interest as data from many real-world cohorts covering different regions and countries were presented for the first time.
Globally, GT1b is the most common subtype of HCV, accounting for 20–25% of all infections. It is the most common GT in Europe, while GT1a prevails in America.3 Phase III RCTs of 8, 12, 16, and 24-week regimens that are approved or soon to be approved have shown high rates of sustained virological response (SVR) for treatment of GT1-infected patients.4-9
HCV TARGET represents a consortium of academic and community medical centres in North America, Germany, and Israel, conducting a longitudinal, observational study of HCV treatment that included simeprevir (SMV)/sofosbuvir (SOF); daclatasvir (DCV)/SOF; ledipasvir (LDV)/SOF; and also ombitasvir (OBV)/paritaprevir (PTV) and dasabuvir (DSV) regimens. A recent analysis within TARGET showed similar results to Phase III trials where most regimens produced uniformly high SVR rates in this real-world cohort (48% in SOF+ribavirin [RBV] regimen, 93–97% for the remaining regimens). Adverse events and discontinuations were infrequent, and the majority were moderate or mild in severity. The patient population in this study comprised a cohort of GT1b-infected patients that started treatment with interferon (IFN)-free regimens prior to August 2015, where 56% of patients had cirrhosis and 59% were treatment-experienced.10
The ABACUS study gave real-life SVR rates in patients with compensated cirrhosis of GT1a: 93%, and GT1b: 96% (intention-to-treat population), which were comparable to those reported in Phase III studies (TURQUOISE-II [GT1a: 95%; GT1b: 99%] and III [GT1b: 100%]).11,12 This Italian national compassionate use programme for early access to HCV therapy for patients recruited >1,000 participants from 176 sites across Italy.13 The primary efficacy for this analysis is based on data from 762 GT1b-infected patients with compensated cirrhosis. The majority of these patients were treatment-experienced and received OBV/PTV/ritonavir (r)+DSV+RBV for 24 (GT1a) or 12 weeks (GT1b).
In Spain, a retrospective, multicentre, non-randomised, prospective data analysis of 1,635 patients again showed high SVR data after treatment with OBV+PTV/r+DSV+RBV. Adverse events were reported in only 1.8% of the patients treated with this ‘3D’ regimen. Hepatic decompensation was reported in seven patients; six patients died, three of these cases attributable to liver failure. In this patient population, almost 50% of participants had liver cirrhosis (Child–Pugh [CP] A and CP-B); in addition, severe adverse events (SAEs) were reported in 72 patients.14
In France, real-world data from the observational ANRS CO22 HEPATHER cohort gave optimal SVR rates for GT1a-infected patients with SMV+SOF for 24 weeks, while SVR rates for GT1b-infected patients were optimal with SMV+SOF+RBV for 12 weeks.15 Overall, the response rates were in line with Phase III data. This study included 15,000 patients with HCV with an 8-year follow-up across 32 centres. There were 552 patients with GT1 or GT4 treated with SMV+SOF±RBV for 12 or 24 weeks; >70% of patients had cirrhosis and were treatment-experienced.
Overall, the real-world data confirm the efficacy and safety of the regimens used for treatment of GT1. For GT4, however, there were limited Phase III data. Infection with GT4 is a real problem in immigrant populations in various countries, and it is also rising in Southern Europe with a prevalence of ≤18%.16 Efficacy data in the real-world cohort may be more important with GT4 than other HCV subtypes due to the enormous variability within the subtype.
High SVR rates with good safety profiles were achieved in GT4-infected patients across PEARL-1 (OBV+PTV/r+RBV), open-label (LDV/SOF), and NEUTRINO (IFN/RBV+SOF) trials, as well as the pooled Phase II/III analysis (elbasvir [EBR]/grazoprevir [GZR]±RBV). However, all these cohorts had <100 patients per trial.17-20 Data from three real-world studies have included GT4-infected patients from France, Egypt, and Qatar. In the Qatar study, 90% (28 out of 31) of patients with compensated cirrhosis and 100% (4 out of 4) with CP-B cirrhosis had undetectable viral load at Week 2 of treatment with OBV/PTV/r±RBV.21
As previously mentioned, real-world SVR rates for GT1 or GT4-infected patients (N=552) analysed in the French ANRS cohort were optimal with SMV+SOF+RBV for 12 or 24 weeks.15 In Egypt, 547 GT4-infected patients were treated with SOF+pegylated IFN/RBV (12 weeks); all patients had advanced fibrosis, while 52% had cirrhosis and 32% were treatment-experienced. An SVR rate of <80% was observed in the treatment-experienced group, which may have been associated with old age, male gender, higher BMI, and cirrhosis.22
Another important topic is the global prevalence of RAV of HCV. Non-structural protein 5A (NS5A) RAV are prevalent worldwide and, as Phase III trials have shown, these variants can be detected in patients who have never been exposed to an antiviral drug before. Sensitive assays show that in most regions about 25% of patients already have naturally occurring substitutions in the NS5A genome, which may lead to a lower susceptibility to NS5A inhibitors. While many patients with RAV on DAA treatment showed high SVR rates, others with NS5A RAV had decreased virological response to some of the regimens (e.g. LDV/SOF±RBV23 or EBR/GZR+RBV).9 If these RAV are induced by treatment failure, they may be particularly long lasting. In contrast to RAV induced by protease inhibitors, the RAV induced by NS5A inhibitors do not disappear (except in a small proportion) over time. This effect has been shown for the LDV/SOF regimen where NS5A RAV were detectable up to 2 years after treatment.24 The HCV TARGET trial and another study in Japan showed that baseline RAV influenced SVR rates.25,26 Due to the limited data available, it remains to be seen whether, despite high response rates in controlled conditions, RAV may become a sudden problem in the real world of suboptimal regimens and settings.
Real-World Experience: What Have We Learned About Advanced Liver Disease?
Professor Robert Brown
Liver disease, such as cirrhosis, is more common and develops more rapidly in HCV-positive patients than HCV-negative patients.27 Survival of patients with compensated cirrhosis is significantly longer than in patients with decompensated cirrhosis. About 7% of patients per year will transition from compensated cirrhosis Stage 1 to Stage 2, while between 7% and 10% will move from compensated to rapid decompensation stage. Over 50% of decompensated patients will die each year, and there is currently no US Food and Drug Administration (FDA) or Medical Devices Agency (MDA) approved medication to improve their chances of survival. If the patients do not improve and recompensate, they will have no long-term benefit, unless their condition is linked to transplantation.28
Following successful clinical trials, DAA treatments are now available for patients with HCV and advanced liver disease. Most randomised trial data, such as from TURQUOISE-II, focusses on compensated or CP-A cirrhosis and shows high efficacy and safety for all 3D regimens with RBV. In the TURQUOISE-III safety trial (N=60), RBV was removed for GT1b patients with cirrhosis, yet high SVR rates with no decompensating events were still achieved.12 Pooled data for >1,000 patients with cirrhosis from 12 Phase II/III trials on the 3D regimen±RBV showed that 1.2% of patients decompensated lower than the expected 7% per year during the trial. Lower baseline albumin, prior non-selective beta blocker use for varices, and lower baseline HCV RNA were independently associated with hepatic decompensation events.29 Excellent safety and efficacy are also seen in patients (N=509) with GT1a/b compensated cirrhosis on LDV/SOF+RBV, with SAEs observed in only 3% of patients.30
As mentioned, high SVR rates are achieved for cirrhotic patients on DAAs in trials, however in the real world, clinicians may be more lenient with certain baseline factors such that some patients who might be on the borderline of decompensation will be treated. Efficacy and safety data are still excellent in most of the real-world experiences.
Many real-world cohorts currently include patients with advanced fibrosis or cirrhosis. The AMBER cohort (N=186) in Poland was a heterogeneous group of patients with HCV GT1, 76% of whom had F3/F4 fibrosis. Similar to trial results, these patients, followed for 12 or 24 weeks on OBV/PTV/r±DSV±RBV, once again exhibited low rates of decompensating events (1.6%) but not out of proportion to either what was expected or what was seen in the trial.31
In Israel, 12 sites recruited 661 GT1-infected patients with F3/F4 fibrosis treated with OBV/PTV/r+DSV±RBV. Similar to Phase III trial results, SVR rates of 99% were achieved, with only 1% of patients having hepatic decompensation (half of these continued therapy and achieved SVR).32 The ABACUS study in Italy included a large population of cirrhotic patients and, once again, an SVR rate >95% was observed with very few SAEs and a low rate of decompensation.13
Multiple real-world cohorts in Spain with patients on OBV/PTV/r±DSV±RBV showed excellent SVR rates. In Madrid, only 2% of GT1b-infected or cirrhotic patients (N=823) had SAEs.33 Another retrospective, multicentre analysis in Spain, of GT1 and GT4-infected patients (N=139) with or without fibrosis, showed low rates of SAEs and no hepatic decompensation events.34 A cohort (N=177) in Barcelona also exhibited excellent efficacy and safety results, although with more SAEs (23%), but fewer discontinuations and no decompensation cases during interim analysis.35
In the USA, the observational analysis of >4,000 treatment-naïve veterans with HCV treated in routine medical practice with LDV/SOF±RBV for 8 or 12 weeks revealed slightly lower SVR rates (87–91%) amongst cirrhotic patients. RBV was prescribed at the discretion of the investigator, so it is not known what impact the addition of RBV to a 12-week over a 24-week regimen would have had.36 The US TRIO cohort contained pharmacy data from centres that agreed to participate. Data confirmed that while efficacy of treatment with LDV/SOF >12 weeks was good for cirrhotic or treatment-naïve GT1a/b patients, the addition of RBV and/or the extension of treatment to 24 weeks for treatment-experienced cirrhoticswould be needed to achieve the desired SVR. Prescribing DAAs outside of the FDA-approved labelling had a negative impact on SVR rates.37
An extensive early access programme in Europe provided access to DCV before market authorisation to >7,000 patients in urgent need of HCV treatment and who had no other treatment options. Patients with mixed GTs that had severe liver disease were treated with DCV/SOF±RBV. SVR rates achieved at 24 weeks were high in patients with CP-A cirrhosis.38
While DAAs are well tolerated, with high rates of SVR in the real world and as reported in clinical trials in patients with compensated cirrhosis, none of the mentioned regimens are approved in decompensated cirrhosis. The contraindications are based on either the label or recommendations (Table 1).
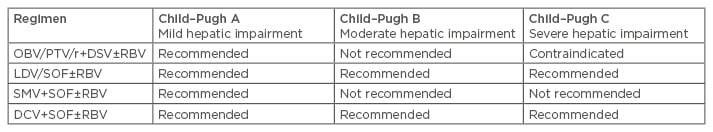
Table 1: Label recommendations for hepatitis C virus genotype-1 infected patients with compensated and decompensated cirrhosis.4,6-8
OBV: ombitasvir; PTV: paritaprevir; r: ritonavir; DSV: dasabuvir; RBV: ribavirin; SOF: sofosbuvir; SMV: simprevir; DCV: daclatasvir; LDV: ledipasvir.
ALLY-1 (DCV+SOF+RBV for 12 weeks)39 and SOLAR-2 (LDV/SOF+RBV for 12/24 weeks)40 trials in patients with decompensated cirrhosis produced good SVR rates in CP-A39 and CP-B, while for CP-C the results are small in number and are variable to suboptimal.39,40
Treatment of HCV GT1 or GT3-infected patients (N=467) with decompensated cirrhosis (however, only 10% CP-C) also highlighted that more data are still required for GT3, particularly for patients with more advanced disease.41 Similar observations have been made from the European early access programme.38 In summary, real-world experience shows good efficacy results for CP-B, while those for CP-C remain questionable.
Real-World Experience: What Have We Learned About Chronic Kidney Disease?
Professor Antonio Craxí
HCV-infected patients have a 23% higher risk of presenting with CKD compared with patients without the virus.42 In a hospital population of US veterans (N=100,518) with HCV, 11.2% had renal impairment with a very high incidence of 16.7/1,000 patient-years.43 Furthermore, in a population where the risk of HCV infection is intrinsically high, such as dialysis patients, the prevalence of HCV is very high (3–68%)44 against the background rate of HCV in that specific population and perhaps for specific countries.
CKD is defined as kidney damage with or without impaired kidney function for ≥3 months with implications for health. Renal function is evaluated using estimated glomerular filtration rate (eGFR), a measure of the flow rate of the kidneys, calculated from the creatinine clearance level (CrCl), and the age, sex, and race of the individual. Renal damage may have many causes; along with HCV, cryoglobulinaemia and nephritis are also quite prevalent causes. Many of the current drugs cannot be used or must be used with caution for patients with severe renal impairment, and renal function may affect the pharmacokinetics and pharmacodynamics of the medication (Table 2).7,8,45-47 HCV seropositivity by itself is also a significant risk factor for proteinuria in the general population.48 A meta-analysis of >800,000 patients from nine different observational studies showed a rather high prevalence of HCV infection, making these patients intrinsically predisposed to have renal damage. In many cases patients with HCV are almost 1.5-times more at risk of having proteinuria than those who are not infected.48 It is therefore very important that physicians recognise how HCV predisposes patients to renal deficiency.
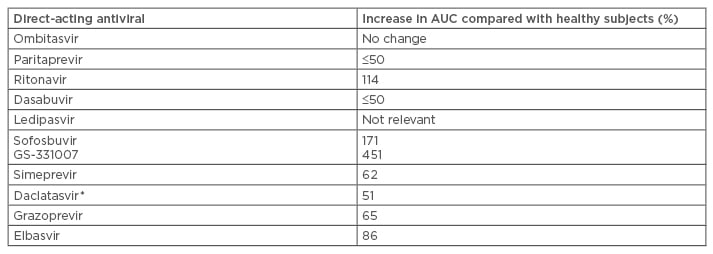
Table 2: Pharmacokinetics of direct-acting antivirals with severe renal impairment (estimated glomerular filtration rate <30 mL/min/1.73 m2).6-8,45,47
*Estimated for creatinine clearance level=15 mL/min
AUC: Area under the curve.
The current American Association for the Study of Liver Diseases (AASLD)49 and EASL guidelines50 state that treatment, particularly in patients with cryoglobulinaemia, is imperative and should be prioritised to avoid organ damage outside the liver. At this stage however, no universal treatment is suggested. Patients on long-term haemodialysis should also be prioritised or considered for HCV therapy due to an increased risk of nosocomial transmission and also because being HCV-negative increases the chances of being considered for a renal transplant.
Currently, regimens approved for use in patients with mild or moderate renal impairment include: OBV/PTV/r+DSV±RBV; LDV/SOF; EBR/GZR; SOF; SMV+SOF; and DCV+SOF. However, patients with severe renal impairment show a significant difference in terms of eGFR and CrCl when treated with drugs such as OBV, LDV, or SOF (rather low SVR rates when combined with RBV). At present, OBV/PTV/r+DSV±RBV and EBR/GZR are the approved treatment options for patients with severe renal impairment and end-stage renal disease (ESRD). These patients, including those on haemodialysis, exhibit up to 95% SVR rates without the use of RBV (RUBY-I study).51 Patients on EBR/GZR showed similar rates (94–99%); however, the frequency of SAEs was rather significant at 14% (C-SURFER study).9,52 These SVR rates are comparable to those observed in non-renal patients.
Following successful clinical trials, there are now numerous national and international cohorts assessing HCV treatments in patients with renal insufficiency (potentially a consequence of liver disease) in the real-world setting. In a Spanish cohort of 100 patients with advanced CKD (37% on dialysis), 47 have shown a promising SVR rate of 89% following treatment with various regimens, with no significant safety problems.53 Another cohort from Spain (N=33) on the 3D regimen with or without a 200 mg dose of RBV produced 100% real-world SVR rates, comparable to those seen in Phase III clinical trials.54 In the ABACUS study in Italy, involving GT1-infected patients with cirrhosis and treated with 3D+RBV, all patients with CrCl <30 mL/min and very low eGFR (i.e. with severe renal deficiency) achieved a high SVR,13 comparable to those reported in clinical trials. In the HCV TARGET, real-world SVR rates of 80–100% were achieved in patients treated with SMV+SOF±RBV across baseline eGFR and independent of renal failure. However, safety data show significant numbers of cases of anaemia when CrCl was low, as well as progressive worsening of renal function with CrCl ≤30 mL/min; similar patterns were observed for adverse events.53
SOF-based regimens have been used widely for patients with HCV as well as those with renal failure. Although not recommended in the summary of product characteristics for SOF, real-world studies in North America and Europe have evaluated SOF-containing regimens in patients with severe renal impairment or ESRD on haemodialysis.55 Two studies from France reported 86–100% SVR rates, although some treatment-related issues have occurred; however, pharmacokinetics of the drug in a patient on dialysis are entirely different from those with renal failure and no dialysis.56,57 In the US and Canada, renal impairment with DAA therapy was also evaluated and compared with that of first-generation protease inhibitors. The first-generation inhibitors cause problems in 25% of patients, regardless of the response rate, while SOF-based regimens caused problems with renal function resulting in renal impairment in 14% of patients.58
While current treatment is effective in a clinical setting, on-treatment renal impairment can occur with some DAA regimens. Safety profiles need to be explored further and in larger cohorts, and need to be carefully considered when treating HCV-infected patients with severe renal impairment.
Question and Answer Session
Professor Ira Jacobson
Q: Does a patient with ESRD, who previously achieved an SVR with treatment, wait for a HCV-negative kidney transplant or be treated right away with a HCV-positive kidney?
A: Using HCV-positive organs in HCV-infected patients regardless of the status will significantly improve the chances of patients with transplants because you can treat these patients. They will get re-infected, possibly with a different strain of HCV, and they can be retreated.
Summary and Close
Professor Ira Jacobson
After almost three decades of clinical trials, a virological cure is now seen in >95% of HCV-infected patients. The medication is effective in a broad range of patients, including those historically considered difficult-to-treat. It is easy to adhere to, has fewer contraindications, and very few serious side effects.
All the real-world studies and registries combine over 43,000 patients, and show that DAAs are well tolerated with very high rates of SVR in clinical practice. However, some populations still have unmet medical needs. These include patients with: RAV; severe renal impairment; GTs 3, 5, and 6; decompensated cirrhosis; DAA failures; and hepatocellular carcinoma. Hepatocellular carcinoma poses a number of issues such as screening, prevention, pathogenesis, and timing of antiviral therapy. The question also remains with regard to compensated cirrhotics: will their improved Model for End-Stage Liver Disease (MELD) scores derail them from their trajectory towards transplantation, yet leave them at the risk of life-threatening complications, and poor quality of life? Another set of patients that could see improved SVR rates are the CP-C group.
Overall, it appears that next-generation regimens will address many of these unmet needs. For example, ASTRAL data show a 95% SVR rate to SOF and velpatasvir for GT3. SURVEYOR-I and II trials present effective pan-genotypic NS5A inhibitors ABT-493 and ABT-530 for patients with or without cirrhosis. Finally, triplet regimens consisting of advanced protease and NS5A inhibitors combined with nucleotide polymerase inhibitors show promise both for initial therapy, with the potential to shorten duration of therapy for many patients, and for re-treatment of patients who have failed to have SVR with first-generation regimens. As this field evolves, the next-generation regimens in HCV will have pan-genotypic efficacy (GT1–6), a high barrier to resistance, efficacy in patients with unmet medical needs, and simple dosing.








