Summary
For patients with the chronic progressive liver disease primary biliary cholangitis (PBC), personalised treatment is key to both preserving liver function and retaining health-related quality of life (HRQoL). This symposium at the 2024 European Association for the Study of the Liver (EASL) congress reimagined ‘PBC’ as standing for a ‘personalised’ approach, managing ‘biochemistry’, and gaining symptom ‘control’ as well as for ‘providing better care’. The symposium started with personal testimony from Mo Christie, a person with PBC, recounting how PBC symptoms, most notably cholestatic pruritus, had severely impacted her HRQoL. This was backed by myriad other patient testimonies collected by the PBC Foundation, of which she is Head of Patient Services. According to symposium chair Gideon Hirshfield, such symptoms necessitate a patient-centric approach to PBC symptom management. While standard treatment for PBC is ursodeoxycholic acid (UDCA), personalised management can help elucidate if, and when, additional therapies are needed to aid disease control. Andreas Kremer and Maria Londoño discussed that this may also take into account risk factors such as liver stiffness, age, and levels of bilirubin and alkaline phosphatase (ALP), all of which may influence progression-free survival rates. David Jones discussed how cholestatic pruritus and/or fatigue can occur in the majority of patients with PBC to some extent and can severely impact HRQoL. Both symptoms need to be discussed and assessed at every visit to a healthcare professional (HCP), with the need for treatment guided by the patient.INTRODUCTION
Gradual destruction of intrahepatic bile ducts is the key characteristic of the chronic, progressive, liver disease PBC.1 This immune-mediated condition occurs predominantly in middle age and has been shown by a number of studies to be 1.6−13.0 times more common in females.2 According to Gideon Hirshfield, “PBC is a disease of great unmet need where real people’s lives are changed and shortened, and their quality of life is reduced.” With this in mind, at the 2024 EASL conference, Hirschfield and the other presenters centred their discussions on how the ‘P’ in PBC should be about taking a ‘personalised,’ patient-centric approach, with the ‘B’ highlighting management of ‘biochemistry’, and the ‘C’ to be for the goal of ‘controlling’ symptoms.
A PATIENT-CENTRIC APPROACH TO TREATING PBC
The Importance of Patient Testimony
According to Mo Christie, a patient’s journey to diagnosis and treatment can vary greatly, with each patient’s experience of PBC being different and complex. As a patient herself, Christie provided personal testimony while also highlighting the experience of fellow patients. She started by showing some of the words patients use to describe feelings related to their symptoms (Figure 1).
Figure 1: Words patients have used to explain how primary biliary cholangitis symptoms make them feel.3
“Symptoms can be debilitating and have a huge impact on quality of life,” said Christie, “yet so many patients tell us that they are not asked about their symptoms at clinic appointments.” This is important because, with regard to a symptom like fatigue, for example, “patients often struggle to share the impact that this has on their quality of life. The words ‘I’m tired’ don’t really come close to describing just how exhausted and debilitated they feel.” Christie also described how another symptom, cholestatic pruritus, “can be extremely difficult for patients to deal with. The extent and impact of this is often not fully understood. A patient once told me that her itch was so bad that she wanted to walk out into the ocean and keep going.”
Christie also shared her own story of how she endured 6 years of different treatments for cholestatic pruritus without success. By this time, she said, “I barely recognised the person I’d become and I rarely left the house. My daughter was then seven and I felt like her childhood was just passing me by.” Eventually, Christie was given two liver transplants, as the first led to complications, and she described how this restored her life.
Christie concluded by discussing how “we need to inform, educate, and empower patients to be involved in their own care journey. Signpost patients to patient organisations (such as the PBC Foundation) where we can help inform, educate, and empower them to be involved in their own care.” Christie finished by appealing to HCPs “to please consider your patient’s individual needs and circumstances. Ask about their symptoms to understand how their quality of life is impacted.”
Moving to Patient-Centric Treatment
The current treatment paradigm for PBC is for all patients to be prescribed 13−15 mg/kg/day UDCA for life. Optimal outcomes of such therapy are normalisation of laboratory parameters and a very low risk of disease progression.1,4 Studies show that with UDCA, 10-year cumulative liver transplantation survival is lower compared to patients not treated with UDCA, even if the response is not complete.5 Of note though, in patients treated with an adequate dose of UDCA for at least 2 years, the likelihood of response is lower in younger patients, males, and those with higher bilirubin and ALP levels.6
Current treatment paradigms typically recommend that initial treatment response is assessed at 6−12 months, at which point, second-line therapy and symptom management are added as required (Figure 2).1,4 This is after ruling out other reasons for liver test abnormalities, such as alcohol consumption, drug-induced liver injury, autoimmune hepatitis, steatotic liver disease, and biliary obstruction.7 Of note though, this recommendation means that second-line treatment is only given if, and when, UDCA treatment fails. A new paradigm suggests more personalised care that assesses baseline risk and then utilises all available therapies from treatment start according to individual results of liver tests, liver fibrosis, symptoms, and risk factors (Figure 2).8 Hirschfield stressed how this patient-centric approach, where symptoms are readily addressed and managed in addition to starting disease-modifying therapy, could make a difference to HRQoL.
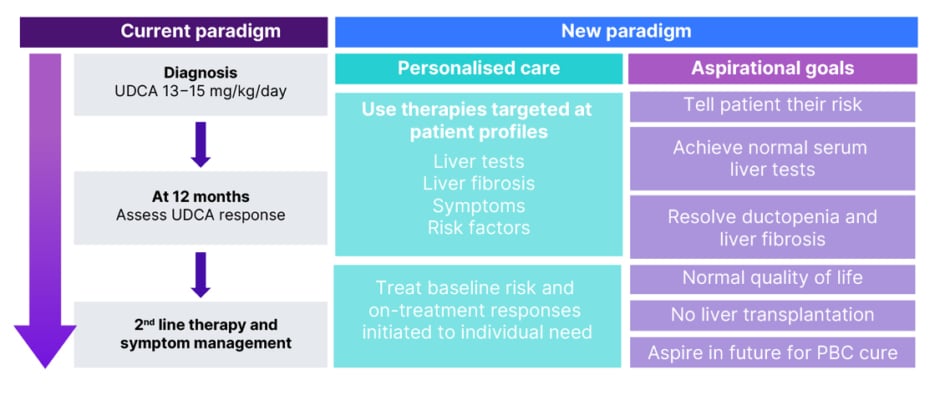
Figure 2: Current versus new treatment paradigms.8
PBC: primary biliary cholangitis; UDCA: ursodeoxycholic acid.
ASSESSING BIOCHEMISTRY AND CONTROLLING SYMPTOMS
Risk stratification for PBC progression can depend on a number of factors including age, sex, symptoms, fibrosis, and biochemical and serological results.1,4 The latter may include increased ALP and bilirubin, decreased albumin, and the presence of anti-gp210.9 Maria Londoño and Andreas Kremer discussed how risk stratification and biochemistry measurements can help guide patient treatment. Risk stratification is important, said Londoño, “because during follow-up, it will help us to make decisions to improve the treatment and care of patients.”
With regard to fibrosis, worse clinical outcomes are typically shown in patients where liver stiffness measurement (LSM) is higher.10 However, variables in outcome according to LSM may also depend on other accompanying factors. A recent study examined the interaction between LSM, ALP, and age in a cohort of 1,047 patients with PBC deemed to have an adequate UDCA response 12 months after initiation. As expected, this study found that 10-year complication-free survival rate was higher for patients with normal ALP levels at study entry (85.7%; 95% CI: 77.5%, 91.0%) compared to those with a higher ALP at this timepoint (73.2%; 95% CI: 61.5%, 81.9%), especially where LSM was ≥10 kPa at study entry (indicating advanced fibrosis).11
With regard to who benefitted most from UDCA therapy, subgroup analysis found that in patients with normal ALP at study entry, the greatest gain in complication-free survival was in the 7.3% of patients who, at study entry, had LSM values ≥10 kPa and were ≤62 years old, with some gains if just one age/LSM value-related condition was met. However, gains were not significant in the 40.7% of participants who, at study entry, were >62 years old and had an LSM value <10 kPa (indicating nonadvanced fibrosis). As such, the authors suggested that “additional therapeutic efforts should be considered in UDCA-treated PBC patients with persistent ALP elevations, particularly in those with advanced disease and/or sufficiently young age.”11
There is also a relationship between ALP and bilirubin levels. For example, an investigation that included over 2,000 patients with PBC showed that, when the cohort was taken as a whole, bilirubin >0.6 times the upper limit of normal (ULN) was predictive of progression to liver transplantation or death. Decreasing bilirubin with UDCA to ≤0.6 x ULN in the first year was associated with a 10.5% improvement in 10-year survival. Of note, if a patient’s ALP levels were normal, survival where bilirubin >0.6 x ULN was similar to when bilirubin was ≤0.6 x ULN. For ALP, 10-year survival was 93.2% where it was ≤1.0 x ULN and 86.1% where it was 1.0−1.67 x ULN. However, in patients with bilirubin >0.6 x ULN, survival for those with ALP 1.0−1.67 x ULN was lower, at 74.2%.12
These results highlight why guidelines suggest that in patients where response to UDCA is inadequate, as shown by ALP >1.67 x ULN or increased bilirubin, there is a high risk of disease progression, and second-line therapy should be considered. This includes obeticholic acid (5 mg/day, increasing to 10 mg/day after 6 months if needed).1,4 Notably, in a recent audit of PBC management in the UK, which included data for 8,968 patients, while 87.7% were receiving first-line treatment with UDCA, 26.7% taking UDCA for ≥12 months had an inadequate response, 48.9% of which were not prescribed second-line therapy, despite guidelines to do so. Other actions that fell short of guidelines included adequate symptom and osteoporosis risk assessments; hepatocellular carcinoma surveillance in patients with cirrhosis; biopsy for PBC/autoimmune hepatitis overlap; and referral and discussion around the possibility of a transplant.13
While treatment in the two scenarios of optimal management or inadequate response is fairly clear-cut, Kremer explained that what is less clear-cut is how to manage patients with an adequate, but not optimal, response (e.g., slightly elevated aspartate transaminase but normal bilirubin), who may be at low-to-moderate risk for disease progression, but may have additional risk factors such as advanced fibrosis, age <50 years old, and be anti-gp210 positive.9,11 As such, with regard to disease management and progression, Kremer stressed how “risk stratification is essential not only at the beginning but also after 1 year of therapy to optimise PBC care. Keep in mind,” he continued, “age and signs of advanced fibrosis as being very important additional factors.”
Kremer concluded that “the decision for additional therapies should not only be based on the biochemical response but on other parameters, age in particular.” However, he cautioned to “always consider a risk-benefit for additional therapies.”
ADDRESSING CHOLESTATIC PRURITUS AND FATIGUE IN PBC
By assessing symptoms that may be overlooked in patients with PBC, most notably cholestatic pruritus and fatigue, Hirschfield stressed how “there’s more opportunity to change what happens to our patients than just how long they live.”
Cholestatic Pruritus
In one USA-based survey, cholestatic pruritus was found to be experienced by 80.5% of 211 patients, of which 37.1% experienced pruritus to a clinically significant degree.14 However, David Jones said, “the symptom of itch is never a symptom in isolation, it is at the epicentre of a whole range of things that really impact people.” Hirschfield added that “when we start off with itch and then broaden it out to the consequences including fatigue, we need to recognise what that means for sleep quality, daytime somnolence, energy, willingness and ability to do physical activity, and impacts on family, jobs, and mood.”
Indeed, in the above-mentioned USA survey, pruritus impacts were shown on several HRQoL domains including emotional, social, educational, domestic, and cognitive factors, as well as on levels of fatigue.14 The latter may be linked to findings that cholestatic pruritus can interfere with sleep, as reported by 74.1% of 162 patients with PBC in another USA survey.15 This is of interest as one meta-analysis investigating the sequelae of low sleep quality in people with multiple underlying medical conditions found significant correlation with occurrences of depression and anxiety, along with worse disease-specific HRQoL scores.16 These relationships are illustrated in Figure 3.
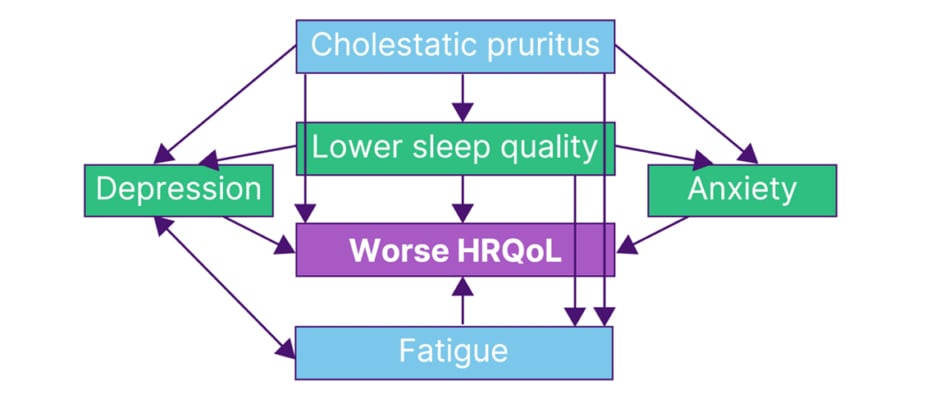
Figure 3: Relationships between cholestatic pruritus, fatigue, and health-related quality of life.14-18
HRQoL: health-related quality of life.
“What we find though,” said Jones, “is that clinicians don’t talk about itch nearly as much as patients perceive it to be a problem.” Indeed, a UK-based survey of 633 patients found that 44.2% were not asked about itch at their last HCP visit,19 with the above USA survey showing that 69.8% of 149 patients said their doctor did not evaluate pruritus occurrence. Of those who were evaluated, this was primarily through questioning only, with very few being formally assessed.15
Such measures include a simple numerical rating scale or visual analogue scale, where a patient is asked to rate their pruritus from absent (0) to worse possible itch (10);20 the Patient Global Impression of Severity Scale-Itch, which rates pruritus from absent to severe;21 and tracker applications for pruritus occurrence and severity that can be noted on paper, or used on a computer or a phone. These, Jones suggested, can also be used to evaluate treatment response. Additionally, he stressed, “don’t just ask for severity, look at the impact on day-to-day life. Is the itch bad enough for you to want to have treatment for it? Are you getting itch at night that is disturbing your sleep? Leave the treatment decision in the hands of the patient,” he also suggested, “if they feel it needs treating, then it needs treating.”
Jones continued by saying that “itch may not be the commonest symptom in PBC, that’s fatigue, but it’s the one that we can make the biggest impact on. If you can treat the itch effectively, and reduce sleep impairment, you will have impacts on all these other symptoms (Figure 3), it’s the key to unlocking the door of symptom control.”
Despite the knowledge of pruritus being a symptom of PBC,14 and that UDCA does not necessarily alleviate cholestatic pruritus,22 one of the USA surveys above found that of those with clinically significant pruritus, one-third never received treatment aimed at this symptom.14 According to EASL and American Association for the Study of Liver Diseases (ASLD) guidelines, first-line treatment for pruritus is bile acid sequestrants. However, Jones cautioned that patients may not tolerate this therapy, as confirmed by Christie. Where pruritus is refractory, rifampicin may be tried as second-line treatment, followed by oral opioid antagonists, then selective serotonin reuptake inhibitors. For those where pruritus is still refractory, clinical trial entry or liver transplantation may be considered.1,23
“My advice,” said Jones, ”is to work through these therapies in a systematic way and make sure you don’t discard therapies before you’ve adequately tried them.” He stressed that a few weeks may be needed for some therapies before response can be fully ascertained. With all treatments, there is a need for a balance between treatment benefits and safety.
However, even with these guidelines,1,23 the USA study showed that only 25.0% of patients with clinically significant pruritus were currently receiving bile acid sequestrants and only 45.2% had ever received this treatment. Respective figures for antihistamines, which have not been proven to reduce cholestatic pruritus, were 65.6% and 64.3%.14 In a UK-based study, 73.5% of 2,194 patients experienced pruritus. Of the 759 where this was persistent, 37.4% were treated with cholestyramine, 11.1% with rifampicin, and 4.5% with naltrexone. Corresponding figures for the 257 patients with severe pruritus were higher, at 50.2%, 23.0%, and 9.0%, but notably did not include everyone with this level of pruritus.20
Other recommendations for patients with cholestatic pruritus include applying moisturisers and appropriate topical agents; bathing with tepid water or using ice packs or wet cooling, moist wraps; avoiding tight or sticky clothing and hot environments; having controlled ultraviolet exposure and trimming nails. Additionally, behavioural measures and relaxation techniques may help stop the itch-scratch-itch cycle.24
Jones concluded that it is important that pruritus is discussed at every visit so that patients know that what they are experiencing may be related to PBC. The discussion should include assessment of whether the pruritus is PBC-related, how severe the pruritus is, how often it occurs, and how pruritus impacts HRQoL.
Fatigue
Another symptom experienced with PBC is mild−severe fatigue, experienced by 93% of patients in one study.25 This symptom can impact a patient’s HRQoL (Figure 3) in domains such as job performance, family and social life, physical activity, mood, sleep, and daytime somnolence.14,17,18,26 Where fatigue is present and includes cognitive deficits, there may be a higher degree of HRQoL impairment compared to patients with fatigue but without cognitive impairment, or those with no or mild fatigue.27
Interviews with patients with PBC and fatigue reflect these findings. They include one person who reported how “I felt I had ‘dumbed down’ and no longer expected so much of myself,” and explained how “my social life deteriorated” and that “I did not like to plan ahead, as I was never sure how much I would be able to manage.” This patient also reported that, while they often mentioned fatigue to their hospital doctors, some were sympathetic but did not offer any treatment, while others “felt I should pull myself together” and that, “as my liver function tests were quite good, this could not really be a problem related to my liver disease.”28 This last point highlights how the degree of fatigue does not necessarily correlate with disease severity. As such, fatigue should be regularly measured in all patients with PBC to properly assess disease-related burden.1,23,29
Indeed, Jones discussed how, even though a patient may feel uncomfortable discussing fatigue, it needs to be assessed at every visit and the patient reassured that many people with PBC experience the same symptom. Support and empathy are key.17 Discussion can include how it is impacting a person’s daily life, in terms of asking questions such as “what do you struggle with?” and the relative severity of fatigue over time. Jones also suggested to emphasise how it was important for patients to maintain social structure and ensure that they left the consultation feeling positive, “even if this is just about them taking ownership of their problems.” He additionally discussed how in his practice, “we have a specialist nurse who spends a lot of time talking to people about this; this is about giving people control of their destiny.”
Fatigue may be rated by both patients and HCPs using a variety of instruments, such as visual analogue scales,30 the Fatigue Assessment Instrument,31 and the fatigue domain of the PBC-40 assessment tool. In the latter, patients are asked to rate how often statements such as ‘I had to force myself to get out of bed,’ ‘I had to have a sleep during the day,’ and ‘fatigue interfered with my daily routine,’ applied to them in the last 4 weeks.32
Of note, use of the PROMIS® fatigue instrument33 in one study confirmed that mean fatigue scores increase with pruritus intensity.14
Fatigue may not be alleviated with UDCA22 and Jones concluded by saying that while “at this moment, we do not have a pharmacological treatment that will improve fatigue; that doesn’t mean we can’t help patients. In particular, we can help them to understand the symptoms and to live the best life they can with it; it’s about helping people to cope.”
CONCLUSION
In conclusion, this symposium underlined how disparities in access to high-quality, patient-centred care for people with PBC need to be addressed. Treatment paradigms should be based on a personalised approach, with individual risks assessed at baseline. They should aspire to both normalise liver function and prevent end-stage liver disease, as well as to address HRQoL. Above all, with symptoms such as fatigue and pruritus, it is important that the patient feels believed and that their concerns are taken seriously. Hirschfield concluded by saying that opportunities for the future for people with PBC are rooted in ‘providing better care’. He urged that, although care has excelled in recent years, the journey isn’t finished. There is a need to continue to improve and strive, and keep changing the focus as more options for patients arise that can be utilised to help optimise as many aspects of PBC-related care as possible.







