Presenters: Vlad Ratziu,1 Yusuf Yilmaz,2 Don Lazas,3 Scott L. Friedman,4 Liat Hayardeny,5 Shaul Kadosh,6 Tali Gorfine,5 Arun J. Sanyal7
1. Sorbonne Université, Institute for Cardiometabolism and Nutrition (ICAN), Hôpital Pitié-Salpêtrière, INSERM UMRS 1138 CRC, Paris, France
2. Liver Research Unit, Institute of Gastroenterology, Marmara University, and Department of Gastroenterology, School of Medicine, Marmara University, Istanbul, Turkey
3. ObjectiveGI, Inc., Nashville, Tennessee, USA
4. Division of Liver Diseases, Icahn School of Medicine at Mount Sinai, New York, USA
5. Galmed Pharmaceuticals Ltd., Tel Aviv, Israel
6. Statexcellence Ltd., Israel
7. Department of Gastroenterology, Virginia Commonwealth University, Richmond, Virginia, USA
Disclosure: Ratziu and Sanyal are Galmed consultants and co-Principal Investigators in the Galmed-sponsored ARMOR study. Yilmaz and Lazas are Investigators in the Galmed-sponsored ARMOR study. Friedman is a Galmed consultant. Hayardeny and Gorfine are Galmed employees. Kadosh is the Galmed statistician.
Acknowledgments: This report was written by Jenny Lloyd, Compass Medical Communications, Ltd., UK.
Support: The publication of this article was funded by Galmed Pharmaceuticals. The content was reviewed by Galmed Pharmaceuticals and authors of the posters for medical and scientific accuracy.
Citation: EMJ Hepatol. 2022;10[Suppl 1]:2-8.
Summary
This article reviews two Aramchol™ posters that were presented at the ‘Liver Meeting®’ 2021 by Vlad Ratziu et al., as well as a presentation given by Ratziu that detailed further study results and their implications. The first poster presented the novel study design of the open-label part of the ARMOR study, in which 150 patients are being randomised to biopsies at 24, 48, or 72 weeks after initiating Aramchol 300 mg twice daily (BID). The second poster, which was accepted as a late-breaking poster presentation, described the results from the first 20 patients with Stage F1–F3 fibrosis (n=20 cohort) who received Aramchol, and had already undergone their scheduled post-baseline biopsy. Data revealed that 60.0% of the 20 patients treated with Aramchol 300 mg BID had fibrosis improvement of ≥1 stage. In the presentation, Ratziu revealed biomarker data from the n=20 cohort, as well as from a larger cohort of patients who had started treatment but had not yet undergone their scheduled biopsy (n=139 cohort). The data showed significant reductions in alanine aminotransferase (ALT), aspartate aminotransferase (AST), fibrosis-4 (Fib-4), and procollagen 3 N-terminal propeptide (ProC-3) in both cohorts. As Fib-4 is a well-known marker of fibrosis and ProC-3 is an emerging biomarker of established fibrosis and active fibrogenesis, the similarities in the biomarker results in the two cohorts indicate that the n=139 cohort data are likely to show a similar, substantial effect on fibrosis improvement as in the n=20 cohort.Introduction
Non-alcoholic steatohepatitis (NASH) is the advanced form of non-alcoholic fatty liver disease, which can lead to further liver damage, advanced fibrosis, cirrhosis, and hepatocellular carcinoma.
Aramchol is a novel fatty acid–bile acid conjugate targeted to the liver that induces beneficial modulation of intrahepatic lipid metabolism. Aramchol is a partial inhibitor of hepatic stearoyl-coenzyme A desaturase 1, with direct anti-fibrotic activity demonstrated in pre-clinical models.1
In a 52-week Phase IIb study, improvement in fibrosis by ≥1 stage without worsening of NASH was observed in 17.5%, 21.3%, and 29.5% of patients in the placebo, Aramchol 400 mg, and Aramchol 600 mg groups, respectively.2 Based on the dose response pattern identified in the Phase IIb study, an increase in exposure offers the potential for greater efficacy in the treatment of NASH. Splitting the dose of Aramchol to 300 mg BID has been shown to increase exposure substantially (by 53%),3 and was therefore selected as the dose regimen for the currently ongoing Phase III study in patients with NASH and fibrosis (ARMOR4).
A Novel Study Design with Aramchol Exploring the Kinetics and Inter-relations of Histological Outcome Measures and Non-invasive Tests Associated with Non-alcoholic Steatohepatitis and Fibrosis for Variable Treatment Durations5
The kinetics of histological changes in patients treated for NASH and fibrosis have not been characterised, yet they are important to understand the timing of optimal treatment effect. NASH studies with different drugs and mechanism of actions have been performed using various exposure durations. Phase II Aramchol studies suggest that favourable effects may be observed as early as 24 weeks, whereas studies of other drugs in this field have continued for up to 72 weeks. No study has examined the kinetics and magnitude of the effect over different treatment durations. Non-invasive tests (NITs) associated with NASH and fibrosis have not yet been validated, and their predictive strength may also depend on treatment duration.
The open-label part of the ARMOR study was designed to provide data on the new dosing regimen with higher exposure, as well as to characterise the kinetics of histological outcomes and NITs for Aramchol.
A total of 150 patients are being enrolled to receive Aramchol 300 mg BID, randomised 1:1:1 to perform a post-baseline liver biopsy at Weeks 24, 48, or 72 (Figure 1). The primary efficacy endpoints are the kinetics of fibrosis improvement without worsening of NASH and NASH resolution without worsening of fibrosis for the different treatment durations. Ratziu emphasised the importance and robustness of the central pathology reading process. Based on guidance from the U.S. Food and Drug Administration (FDA), all biopsies are being examined by three experienced liver pathologists, first individually and then by consensus.
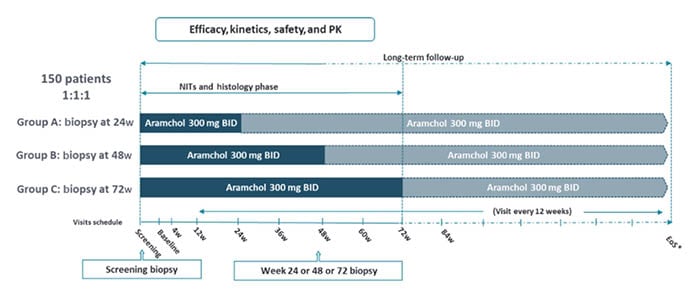
Figure 1: Study design.6
*EoS will be aligned with the double-blind part. A second post-baseline liver biopsy will be collected for fibrosis non-responders.
BID: twice daily; EoS: end of study; NITs: non-invasive tests; PK: pharmacokinetics; w: weeks.
A second post-baseline liver biopsy in non-responders is being performed to allow identification of potential delayed responders versus early responders to Aramchol.
A double-blind, randomised part, in which approximately 2,000 patients with NASH will be randomised 2:1 to Aramchol meglumine (a new improved formulation) or placebo, will follow. The sample size and duration for the histology-based interim analysis to support regulatory submission will be further discussed with the FDA, following initial results revealed herein.
Efficacy of Higher Daily Aramchol Dose on Fibrosis Improvement in the ARMOR Study Open-Label Part6
Results from the first 20 patients with F1–F3 fibrosis who received Aramchol 300 mg BID, in whom the scheduled post-baseline biopsy has been performed, were presented as a late-breaking poster at the American Association for the Study of Liver Diseases (AASLD) (n=20 cohort). Altogether, 12 of 20 patients (60.0%) showed fibrosis improvement of ≥1 stage (five of nine after 24 weeks; six of nine after 48 weeks; and one of two after 72 weeks). In five patients (25.0%), fibrosis was reduced by 2 points, and seven patients (35.0%) showed 1-point improvement (Figure 2A). Among the remaining eight patients, seven (35.0%) had stable fibrosis, and one (5.0%) had worsened fibrosis. Put another way, the proportion of patients with advanced fibrosis (Stage F3) reduced from 65.0% at baseline to 25.0% after Aramchol, while the proportion with F1 or F0 increased from 15.0% to 45.0% (Figures 2B and 2C). Further analysis showed that in nine of 20 patients (45.0%), fibrosis improved without worsening of NASH. A total of 19 patients were available for assessing the NASH resolution endpoint, of which five (26.3%) showed NASH resolution without worsening of fibrosis.
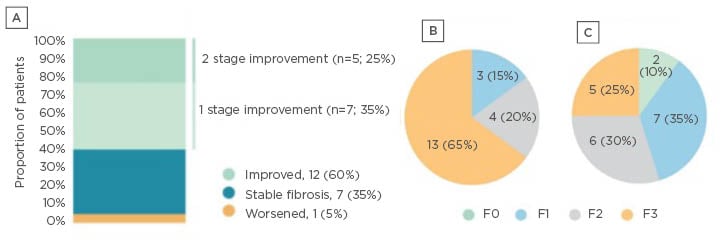
Figure 2: Effect of AramcholTM on fibrosis stage in the n=20 cohort.
A) Change from baseline; B) fibrosis stage at baseline; and C) fibrosis stage after treatment.6
In a separate talk, Ratziu described the overall response rate of 60.0% as “very impressive,” given that placebo response rates in other trials are around 15–30%. He also responded to a question on the importance of knowing how quickly patients respond to Aramchol, and what impact this could have for real-world use. Some drugs act quickly, and others more slowly. Most studies in this field only test at one fixed time point (although the CENTAUR trial7 of cenicriviroc used serial biopsies, at 1 and 2 years, to study when responses occurred). With one time point, it is impossible to know whether an effect is just starting or has reached a maximum. Therefore, in ARMOR, biopsies are being carried out at three time points to ascertain how long patients take to respond to Aramchol. Although there will be some interpatient variability, if maximum response levels are seen at 48 weeks, this can guide the optimal timing of the interim analysis. Regarding real-world use, treatment is likely to be needed long-term, but it is still advantageous to know when a benefit may occur.
In response to an important question about how these fibrosis improvement results compare to other recent data in the field, Ratziu said that comparisons between studies should be undertaken with caution, as they have different populations, designs, durations, etc. However, based on the results to date (from ARREST2 and ARMOR), Aramchol appears to have a more pronounced effect on fibrosis than semaglutide,8 resmetiron,9 or obeticholic acid.10 Obviously, full Phase III data are required before any firm conclusions can be reached, but this is very encouraging, as drugs that reduce fibrosis are needed, and very few have an anti-fibrotic effect. Although the results from the open-label part of ARMOR have no placebo control, placebo response rates are typically much lower than the 60% response seen with Aramchol.
Clinical Antifibrotic Effect of Aramchol: Histology and Convergent Biomarker Data
In a Galmed-sponsored call, Ratziu reviewed further study results and their implications, including data from a larger cohort (n=139 cohort), which was analysed for specific NITs associated with liver fibrosis. The larger cohort is composed of the first 20 patients for which the biopsy results have been described, and an additional 119 patients who have started treatment, most of whom have some NIT results available. As is to be expected in an ongoing study, data are available for individual patients for different treatment durations, according to their length of participation in the study. The statistical model used for analysis was a baseline-adjusted mixed model for repeated measures analysis. Statistically significant reductions in ALT, AST, and Fib-4 were demonstrated in both the smaller and larger cohorts (Table 1; Figure 3A-C).
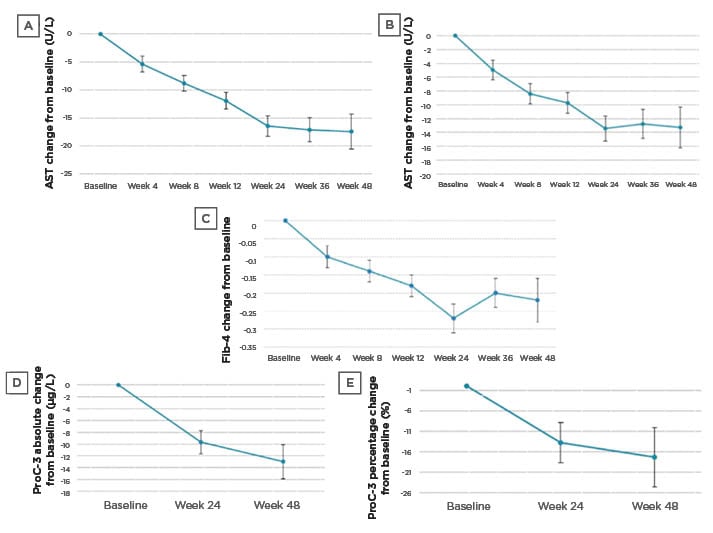
Figure 3: Effect of AramcholTM on biomarkers in the n=139 cohort.
A) ALT change from baseline; B) AST change from baseline; C) Fib-4 change from baseline; D) ProC-3 absolute change from baseline; and E) ProC-3 percentage change from baseline.
Error bars are standard errors.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; Fib-4: fibrosis-4; ProC-3: procollagen 3 N-terminal propeptide.
ProC-3 values were analysed by Nordic Bioscience (Herlev, Denmark), using an improved methodology with higher sensitivity and specificity. Data for both cohorts (n=19 and n=43 with Week 24 ProC-3 data) showed statistically significant reductions when looking at absolute or relative changes from baseline (which was a mean of 47.2 μg/L in the larger cohort) (Table 1; Figure 3D-E).
Ratziu concluded that overall, 60.0% of patients in the n=20 cohort had reductions in fibrosis at biopsy, and there were significant and sustained reductions in all four biomarkers (ALT, AST, Fib-4, and ProC-3) at 24 and 48 weeks in the n=20 cohort and the n=139 cohort. Together with the findings from the Phase IIb ARREST study, these results reinforce the optimism that Aramchol will be able to induce histological improvement, particularly fibrosis reversal. They also provide support to the hypothesis that higher exposure will result in improved clinical benefit.
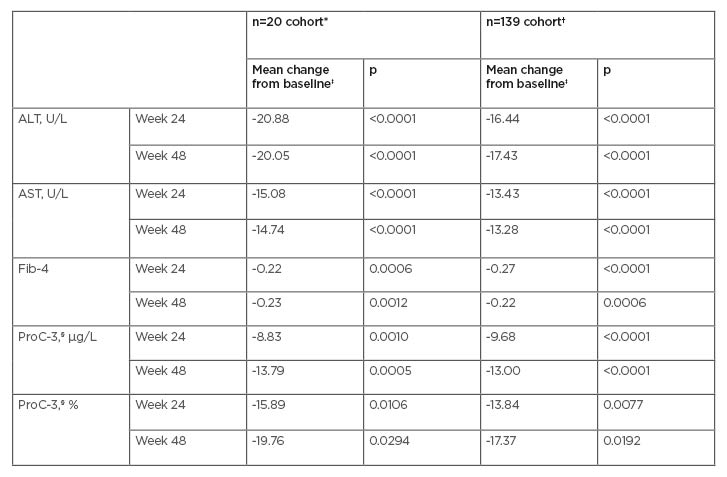
Table 1: Effect of AramcholTM on biomarkers in the n=20 cohort and the n=139 cohort.
*Numbers of patients in the n=20 cohort at Weeks 24 and 48, respectively, are 19 and 9 (ALT, AST, Fib-4) and 19 and 10 (ProC-3).
†Numbers of patients in the n=139 cohort at Weeks 24 and 48, respectively, are 61 and 18 (ALT, AST), 56 and 18 (Fib-4), and 43 and 15 (ProC-3).
‡Mixed model for repeated measures baseline-adjusted analyses.
§ProC-3 levels were analysed by Nordic Bioscience (Herlev, Denmark) using an improved methodology with higher sensitivity and specificity. Mean baseline ProC-3 in the n=139 cohort was 47.2 µg/L.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; Fib-4: fibrosis-4; ProC-3: procollagen 3 N-terminal propeptide.
Allen Baharaff (President and CEO of Galmed) concluded the talk by stating that the current results indicate that Aramchol could be highly beneficial for the treatment of liver fibrosis. The effect of Aramchol on fibrosis has been improved by increasing exposure, and the primary endpoint (fibrosis) is robust, as three pathologists are examining the biopsies. This open-label part of ARMOR was designed specifically to address the question of optimal treatment duration, and the current data reinforce three main points. Firstly, downregulation of hepatic stearoyl-coenzyme A desaturase 1 results in significant improvement in liver fibrosis. The effects of Aramchol on collagen production and fibrosis translate directly into these beneficial results. Sixty percent of patients had an improvement in fibrosis, with only 5% having worsening fibrosis. Secondly, the hypothesis that higher exposure would result in better clinical efficacy appears to be true. This may lead to a shorter duration Phase III trial, and earlier approval of Aramchol. These data will enable an evidence-based discussion with the FDA. Lastly, the biomarker data are similar in the n=20 cohort and the n=139 cohort, which is highly encouraging.

![EMJ Hepatology 10 [Supplement 1] 2022 Feature Image](https://www.emjreviews.com/wp-content/uploads/2022/01/EMJ-Hepatology-10-Supplement-1-2022-Feature-Image-940x563.jpg)






