Abstract
As the rates of obesity increase worldwide, the prevalence of nonalcoholic fatty liver disease (NAFLD) has risen and it is now the most common cause of liver disease in the developed world. A significant proportion of patients with NAFLD develop nonalcoholic steatohepatitis and progressive liver fibrosis, which can lead to cirrhosis and its complications. NAFLD should be suspected in individuals who have central obesity and metabolic risk factors. A diagnosis of NAFLD can be made when patients have evidence of steatosis on imaging or if they have raised liver enzymes with a background of metabolic risk factors, provided other causes of liver disease and excessive alcohol consumption are excluded. Making a specific diagnosis of NAFLD is important so that affected individuals can receive specific treatment and be monitored for its complications. The stage of liver fibrosis is the most important prognostic factor so must be assessed in all patients; a number of simple blood tests and imaging modalities allow accurate fibrosis staging without the need for liver biopsy. The aim of this narrative review is to provide a practical overview relating to the diagnosis and staging of NAFLD using noninvasive tests that are widely available in primary and secondary care.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a disorder characterised by central obesity-associated fat deposition in the liver.1 With rising obesity rates worldwide, NAFLD has become the most common liver disease, affecting 17–46% of adults in Western countries.2 There has also been a significant rise in liver-related complications secondary to NAFLD, such as hepatocellular carcinoma and liver failure, which has resulted in increased liver transplant rates and hospital admissions for patients with cirrhosis complications.3,4
NAFLD is defined as steatosis affecting >5% of hepatocytes in the absence of a secondary cause, such as the use of steatogenic drugs or harmful levels of alcohol consumption (typically defined as >20 g/day for women and >30 g/day for men).2 NAFLD is strongly associated with central obesity and metabolic syndrome; almost all individuals with NAFLD have one or more features of metabolic syndrome.5 Although most individuals with NAFLD have a BMI that lies in the overweight or obese category, 7% of cases have a normal weight BMI (<25 kg/m2) but they will usually have evidence of central adiposity.6
NAFLD is an umbrella term covering the full spectrum of fatty liver disease, which includes steatosis only (fat without hepatocellular injury; also termed nonalcoholic fatty liver), nonalcoholic steatohepatitis (NASH; fat with hepatocellular injury and inflammation with or without fibrosis), and cirrhosis.7 Overall, 10–30% of the NAFLD population have NASH,8,9 and this can progress to cirrhosis. Individuals who have steatosis only, and do not progress to NASH, have a good long-term prognosis with very low rates of advanced liver disease.10 However, approximately 40% of individuals with steatosis develop NASH in the medium-term (5–10 years) and are at risk of progression to advanced liver disease.11 Progression is more likely to occur if individuals have diabetes or develop diabetes, or if their body weight progressively increases, with a central pattern of adiposity. Overall, in secondary care cohorts of patients with NAFLD, 25–40% will develop progressive liver fibrosis, ultimately leading to cirrhosis in 10–20% of patients.11,12
It has recently been established that the most important predictor of mortality in individuals with NAFLD is the stage of liver fibrosis.13 Individuals with advanced fibrosis (F3; bridging fibrosis) or cirrhosis (F4) have a significantly increased risk of short-to-medium-term all-cause mortality compared with a control population.14 Patients with F2 fibrosis (periportal or portal fibrosis) are at an increased risk of mortality in the longer term (>20 years), whereas those with F0–1 fibrosis are not at an increased risk of mortality. Therefore, it is particularly important for clinicians to identify individuals with advanced fibrosis and cirrhosis so they can be screened for complications and managed aggressively to reduce their risk of further progression. It is also important to identify those with F2 fibrosis to try and prevent progression to more advanced liver disease.
Currently, the mainstay of treatment for NAFLD is lifestyle modification aimed at weight loss and increasing activity levels.15 Weight loss of >5% is associated with improvements in steatosis and inflammation, and >10% weight loss may also reduce liver fibrosis.16 All forms of exercise (moderate intensity, high intensity, or resistance) can improve steatosis independent of weight loss.17 There is also evidence from small trials that treatment with high-dose vitamin E (800 IU/day), pioglitazone, or liraglutide improves NASH in some patients, and these treatments are currently available (albeit not licensed for NAFLD) so can be used to treat individuals with NAFLD who are at a high risk of disease progression.1,2,18 Moreover, several new drugs are currently in Phase III trials,19-22 having shown positive effects in individuals with NASH in Phase II trials, so it is likely that drugs will be licensed for NAFLD within the next 5 years.
Given the large proportion of individuals with NAFLD in the community, it is important that physicians in primary and secondary care have a robust process in place to diagnose and stage NAFLD. This is particularly critical for identifying individuals with more advanced NAFLD to ensure they receive appropriate treatment and monitoring. The aim of this review is to provide a practical overview relating to the diagnosis and staging of NAFLD using noninvasive tests that are widely available in primary and secondary care. The review will also discuss those at risk of NAFLD and how to diagnose and stage the disease.
WHO IS AT RISK OF NONALCOHOLIC FATTY LIVER DISEASE?
NAFLD is strongly associated with increased body weight and metabolic syndrome; therefore, using BMI, waist circumference, or assessment of metabolic risk factors can identify individuals at risk of NAFLD. European guidelines recommend that individuals with metabolic risk factors should undergo procedures for the diagnosis of NAFLD.2 Overall, hepatic steatosis is present in >80% of centrally obese individuals and 70–90% of those with Type 2 diabetes mellitus (T2DM).1 Given the high burden of disease in these patients, it is important for clinicians to proactively look for NAFLD. Subjects with T2DM or full metabolic syndrome are particularly at risk of more advanced NAFLD. A recent study screened 1,799 diabetic patients for liver fibrosis using transient elastography (TE) and found that 17% of the cohort had advanced fibrosis or cirrhosis and 11% had cirrhosis.23
DIAGNOSIS OF NONALCOHOLIC FATTY LIVER DISEASE
Most individuals with NAFLD are asymptomatic and present incidentally with raised liver enzymes or evidence of steatosis on imaging;24 if symptoms do occur, they include right upper quadrant pain or fatigue. Although liver enzymes are frequently ‘normal’ in patients with NAFLD, identification of raised liver enzymes often prompts further investigation. The most common liver enzyme abnormality seen in patients with NAFLD is raised gamma-glutamyltransferase (GGT) levels.25 Raised transaminases are also seen, but 80% of individuals with NAFLD have an alanine aminotransferase (ALT) level within the ‘normal’ laboratory range (<35–50 U/L depending on the laboratory).5 The use of a lower normal range for ALT (<30 U/L for males and <19 U/L for females) is more sensitive for NAFLD.26 However, serum ALT values do not correlate with histological findings in NAFLD and, therefore, ALT cannot be used to make the diagnosis, distinguish between steatosis and steatohepatitis, or quantify the stage of fibrosis.27,28 Raised liver enzymes, typically elevated ALT and/or GGT, in an individual with obesity or who has metabolic risk factors usually signifies fatty liver, provided other causes of liver disease are excluded (Box 1),1,24 but clinicians must not rely on liver enzymes to diagnose NAFLD. The finding of a raised serum immunoglobulin A or ferritin with normal transferrin saturation can be supportive of a diagnosis of NAFLD because these abnormalities are present in 46% and 33% of patients with NAFLD, respectively.29
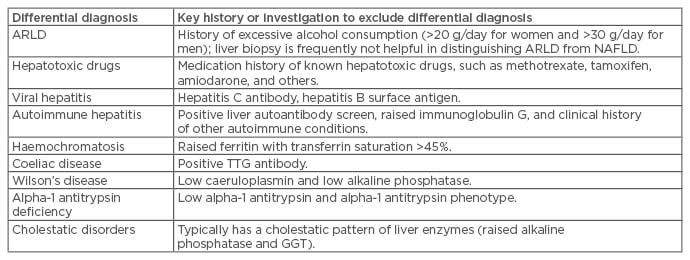
Box 1: Main differential diagnoses that should be excluded when investigating nonalcoholic fatty liver disease.
ARLD: alcohol-related liver disease; GGT: gamma-glutamyltransferase; NAFLD: nonalcoholic fatty liver disease; TTG: tissue transglutaminase.
To be diagnosed with NAFLD, patients must not regularly consume excessive alcohol (>30 g/day for males or 20 g/day for females). In clinical practice, it is common to encounter patients with fatty liver, metabolic risk factors, or central obesity, who also regularly consume more than this amount of alcohol. These individuals are at a particularly high risk of developing advanced liver disease because steatosis from alcohol and metabolic syndrome appears to act synergistically, leading to a more severe liver injury.30 Reducing their alcohol intake as well as managing their obesity and metabolic syndrome is the mainstay of management for these individuals.
Once suspected clinically, the presence of hepatic steatosis should be confirmed on imaging.2 Ultrasound is the first-line investigation for these cases because it is inexpensive and readily available. A fatty liver is associated with increased hepatic echogenicity and appears bright on ultrasound. Ultrasound is accurate for a diagnosis of >20–30% steatosis cases (85% sensitivity and 94% specificity) but is poorly sensitive for mild steatosis.31 There have been recent developments using semiquantitative ultrasound scores to help improve the diagnostic accuracy of ultrasound;32 however, this technique remains operator-dependent and is not routinely available in all clinical settings. Therefore, if clinicians have a strong clinical suspicion that a patient has hepatic steatosis and the ultrasound is negative, then further investigations may be required. In this situation, measurement of controlled attenuation parameter (CAP) can be helpful. This is measured contemporaneously with liver stiffness by TE (FibroscanTM, Echosens, Paris, France), which is available in many secondary care centres. CAP provides an estimate of steatosis by measuring changes in the propagation of a shear wave through the liver, which is altered in a steatotic liver. This technique gives a reading of 100–400 dB/m and has been shown to accurately detect >10% and >33% of steatosis (area under receiver operator curve [AUROC]: 0.91 and 0.95, respectively).33 One study showed that a CAP reading of ≥283 dB/m was 77% accurate for a diagnosis of >10% steatosis.34
Magnetic resonance imaging (MRI) is the most accurate noninvasive modality for diagnosing steatosis but is expensive and not easily accessible. Liver fat can be measured using a number of techniques, including the Dixon technique for in-phase and out-of-phase imaging, magnetic resonance spectroscopy, and MRI-proton density fat fraction (MRI-PDFF).35,36 All of these techniques are quantitative but require specific expertise to interpret the results. MRI-PDFF, the newest technique, has the advantage of assessing steatosis in the whole liver and it has been shown to correlate well with histological assessment of steatosis.36 Moreover, changes in MRI-PDFF following treatment for NAFLD correlate with histological changes in liver fat, suggesting that this technique may be useful to monitor treatment response.37
In most cases, NAFLD can be confidently diagnosed without the need for a liver biopsy in individuals with steatosis on imaging or in those with raised liver enzymes and metabolic risk factors, after the exclusion of other causes (Box 1). In cases where there is diagnostic uncertainty, a liver biopsy is usually helpful to confirm the diagnosis.
STAGING OF NONALCOHOLIC FATTY LIVER DISEASE
Once a diagnosis of NAFLD has been made, it is vital to stage the disease to assess prognosis and determine if specific treatment (in addition to lifestyle changes) for NAFLD is required. As previously discussed, the stage of fibrosis is the most important prognostic factor in NAFLD so should be assessed in all patients. There have been numerous studies assessing the diagnostic accuracy of a multitude of biomarkers of fibrosis in NAFLD;38 however, many of these biomarkers are not widely available or remain under investigation, so discussion here will focus on the routinely available tests.
Simple Noninvasive Fibrosis Scoring Systems
Several simple noninvasive scoring systems for fibrosis have been assessed and validated in NAFLD. These are derived from routinely available laboratory tests (ALT, aspartate transaminase [AST], platelets, or albumin) and clinical measurements (such as age, BMI, and presence of T2DM), and have the advantage of being inexpensive and widely available. Some of the more widely used scoring markers are discussed in Table 1.
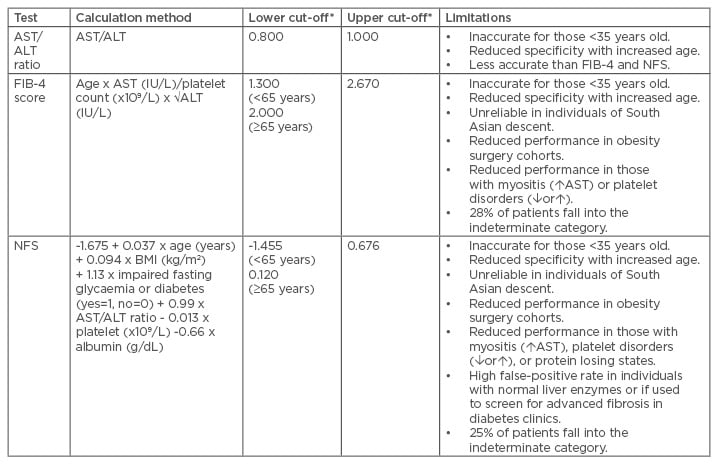
Table 1: An overview of the simple noninvasive fibrosis markers and their limitations.27,39-43
*A score less than the lower cut-off excludes advanced fibrosis and a score above the upper cut-off diagnoses advanced fibrosis. Scores between the lower and upper cut-offs are indeterminate.
ALT: alanine aminotransferase; AST: aspartate transaminase; FIB-4: Fibrosis-4 score; NFS: nonalcoholic fatty liver disease fibrosis score.
Aspartate Transaminase/Alanine Aminotransferase Ratio
The simplest score is the AST/ALT ratio (AAR).39 As fibrosis progresses to advanced fibrosis or cirrhosis, the serum ALT level typically falls, while the AST level remains stable or increases, resulting in an increased AAR.27,44 One study in patients with NAFLD found that an AAR <0.8 could exclude advanced fibrosis with reasonable accuracy (AUROC: 0.83; negative predictive value [NPV]: 93%).27 However, more complex models that incorporate the AAR, such as the Fibrosis-4 (FIB-4) score or NAFLD fibrosis score (NFS), are generally more accurate.
Nonalcoholic Fatty Liver Disease Fibrosis Score
The NFS was developed to diagnose or exclude advanced fibrosis (F3–4) cases in a multicentre cohort of 733 patients with NAFLD.40 The model includes age, BMI, AAR, presence of T2DM or impaired fasting glycaemia, platelet count, and albumin, and can easily be calculated using an online calculator (Table 1). This model uses two diagnostic cut-offs: a score of <-1.455 can exclude advanced fibrosis with reasonable accuracy (NPV: 88–93%), while the higher cut-off of >0.676 can detect advanced fibrosis with some accuracy (positive predictive value: 82–90%).40 Individuals with an indeterminate score usually require a second-line fibrosis assessment. The NFS has been validated in multiple cohorts worldwide41,45-47 and has also been shown to predict mortality (all-cause, liver, and cardiovascular) in patients with NAFLD.48
Fibrosis-4 Score
This score was originally developed in a cohort of individuals with hepatitis C virus/HIV coinfection and is also effective in patients with NAFLD.41,42 The model includes age, ALT, AST, and platelet count (Table 1). A score of <1.3 reliably excludes advanced fibrosis (NPV: 90–95%), while a score of >2.67 is strongly suggestive of advanced fibrosis (positive predictive value: 80%).27,41 Only 28% of patients fall into the unclassified category.27,41 The FIB-4 score has also been externally validated in many cohorts,45-47 including patients with NAFLD and normal ALT levels.49 Overall, the FIB-4 score performed slightly better than the NFS in most studies and requires fewer variables to calculate, therefore making it the most simple noninvasive score of choice in our unit.
BARD Score
This score includes the weighted sum of BMI (>28 kg/m2=1 point), AAR (>0.8=2 points), and the presence of T2DM (present=1 point).44 A score of <2 has an excellent NPV of 95–97% in excluding advanced fibrosis (F3-4); however, the false-positive rate for a score of ≥2 is high, which limits its use in clinical practice.27
Aspartate Transaminase to Platelet Ratio Index
The aspartate transaminase to platelet ratio index has been extensively investigated in patients with hepatitis C virus and a score of >1 is suggestive of cirrhosis. However, the performance of the index was suboptimal (AUROC: 0.67 for diagnosis of F3–4) when compared with other simple noninvasive markers in patients with NAFLD.27
Limitations of Simple Noninvasive Fibrosis Scores in Nonalcoholic Fatty Liver Disease
The NFS and FIB-4 scores are the most widely used simple noninvasive scores and are recommended in European guidelines.2 However, these scores do have some limitations (Table 1), which are important to consider when using them in clinical practice. Firstly, these scores have been derived to exclude or diagnose advanced fibrosis or cirrhosis (F3–4) and have limited ability to detect earlier stages of fibrosis. Both the FIB-4 score and NFS have good NPV and can therefore reliably exclude advanced fibrosis; as a result, their main role is to identify lower risk patients who may not need referral to secondary care.27 However, both scores have a high false-positive rate for advanced fibrosis, so further investigations to confirm advanced fibrosis are often required in those with indeterminate or high scores.
It has been recently appreciated that age is an important factor affecting the accuracy of the FIB-4 score and NFS. One study showed that the NFS and FIB-4 score performed poorly in patients with NAFLD aged <35 years (AUROC: 0.52 and 0.60 for F3 and F4, respectively), probably due to the low prevalence of F3 or F4 scores in that age group.50 Therefore, alternative strategies are needed to stage fibrosis in this age group. In the same study, it was shown that the specificity for advanced fibrosis with the NFS and the FIB-4 scores decreased with age, becoming unacceptably low in those aged >65 years (for F3 and F4, 20% and 35%, respectively, compared to 80% and 91% in patients 36–45 years old, respectively). As a result, new cut-offs have been suggested to reduce the false-positive rate for F3–4 fibrosis in those aged >65 years (<2.0 for FIB-4 and <0.12 for NFS). These cut-offs have been incorporated into UK guidelines.51
It has also been shown that simple noninvasive fibrosis tests perform differently in some ethnic groups. One study found that the NFS and FIB-4 score have reduced sensitivity for significant or advanced fibrosis in individuals of South Asian ethnicity, possibly due to the high rates of obesity and T2DM in this population.52 Alternative diagnostic cut-offs may address this problem. Simple noninvasive fibrosis scores may also have reduced performance in cohorts of patients without known liver disease undergoing obesity surgery.53 Moreover, it remains unknown how well these scores perform in asymptomatic individuals in the community with incidentally identified fatty liver and normal levels of liver enzymes.
Overall, despite their limitations, the FIB-4 score and NFS offer a good first-line test to stage liver fibrosis and if individuals have a low score (using appropriate age corrected cut-offs), advanced fibrosis can reliably be excluded. For those with an indeterminate or high score, it is usually appropriate to conduct a second- line test to confirm advanced fibrosis.
Biomarkers and Commercial Fibrosis Panels
A number of biomarker panels for fibrosis have been studied in patients with NAFLD, with a few used routinely in some centres. Given their expense compared with simple noninvasive tests, biomarker panels are often used as a second-line.
The Enhanced Liver Fibrosis (ELFTM) Test
This commercial panel includes tissue inhibitor of metalloproteinase-1, hyaluronic acid, and amino-terminal propeptide of Type III procollagen and is marginally more effective than the NFS for measuring advanced fibrosis (F3–4) in patients with NAFLD (AUROC: 0.93 versus 0.89).54 An advantage of the ELFTM test (Siemens Healthcare Diagnostics Inc., Tarrytown, New York, USA) over the NFS and FIB-4 score is that it has a single cut-off for advanced fibrosis, which eliminates indeterminate results, but it is expensive when compared with simple fibrosis markers. The National Institute of Health and Care Excellence (NICE) recommends the ELF test first-line to stage fibrosis in patients with NAFLD. However, it is more commonly used as a second-line after simple noninvasive scores.55
FibroTest
The FibroTest is a commercial panel, commonly used in France, including total bilirubin, GGT, α2-macroglobulin, apolipoprotein A1 and haptoglobin, age, and sex. It has been validated in several liver diseases, including NAFLD where it has acceptable accuracy for advanced fibrosis (F3–4; AUROC: 0.81–0.92).56
Imaging Assessment of Fibrosis
Staging of fibrosis with imaging is frequently conducted following an initial triage with simple noninvasive scores or biomarkers. A thorough review of advances in imaging assessment of NAFLD has been recently conducted.57 Here, we will discuss imaging techniques that are widely available.
Transient Elastography
TE (Fibroscan) is the most commonly used imaging modality for liver fibrosis assessment and has been extensively validated.58 TE uses ultrasound to assess liver elasticity by measuring the velocity of a shear wave through a region of interest in the liver. The liver stiffness measurement correlates well with fibrosis stage in a range of liver diseases, including NAFLD. Two probes are available for use in adults: the M probe and the XL probe. Both probes have been validated in patients with NAFLD but give slightly different readings so it is important to use the appropriate diagnostic cut-offs for each probe.59 A limitation of TE is the failure rate, particularly in obese individuals, although this is reduced with the XL probe.59,60
Studies have shown that TE has an excellent ability to exclude advanced fibrosis, with few false-negatives.59 It also has better positive predictive ability for significant and advanced fibrosis than simple noninvasive tests and, therefore, offers a good second-line test to confirm advanced fibrosis.61 Although there is still debate over the optimum cut-off for TE, as a general rule <8.0 kPa (or <7.2 kPa for the XL probe) reliably excludes advanced fibrosis (F3-4) and >9.6 kPa suggests F3-4.57
It has recently been shown that the degree of hepatic steatosis has an impact on the accuracy of TE; one recent study showed that the false-positive rate for ≥F2 or F3–4 was significantly higher in subjects with severe steatosis compared with those with mild or moderate steatosis.62 One way to mitigate against this could be to adjust liver stiffness measurement cut-offs according to the patient’s CAP value,63 but further studies are needed to clearly define the diagnostic cut-offs.
Acoustic Radiation Force Impulse Imaging
Standard ultrasound has a very limited ability to stage liver fibrosis other than diagnosing cirrhosis when features of portal hypertension are present. However, many ultrasound machines can now measure liver elasticity using acoustic radiation force impulse imaging (ARFI), a form of shear wave elastography. There are a few variations of ARFI available, the best studied in NAFLD being Virtual TouchTM Quantification (Siemens Medical Solutions Inc., Mountain View, California, USA). A meta-analysis showed that ARFI was reasonably accurate in diagnosing significant fibrosis (≥F2; AUROC: 0.898).64 However, optimum diagnostic cut-offs have not been determined. Another method of ultrasound elastography, supersonic shear imaging, has also shown promise, performing slightly better than TE and ARFI in a recent study of 291 patients with biopsy-proven NAFLD,65 but further validation is required.
Magnetic Resonance Elastography
Magnetic resonance elastography is considered the most accurate noninvasive test for liver fibrosis in patients with NAFLD.66 This technique measures elasticity in the whole liver using a modified phase-contrast pulse sequence that allows visualisation of a transmitted shear wave in the liver tissue. Although not widely available, studies have shown that magnetic resonance elastography is superior to TE in patients with NAFLD, particularly for the diagnosis of early-stage fibrosis.67,68 This technology is currently being assessed as a potential outcome measure for therapeutic clinical trials.
Biomarkers of Steatohepatitis
Although fibrosis stage is the key factor associated with long-term prognosis, identifying individuals with active steatohepatitis is important because hepatocellular injury leads to the development of fibrosis and the treatment of steatohepatitis reduces the risk of disease progression. Currently, there are no validated biomarkers that can reliably diagnose NASH in an individual patient. One previous study suggested that elevated serum cytokeratin-18 levels, a marker of hepatic apoptosis, may indicate the presence of NASH;69 however, subsequent studies found that cytokeratin-18 levels may not help with diagnosis in individual patients.70 A number of other biomarkers of steatohepatitis are under investigation at present.38 Currently, the only way of reliably diagnosing NASH is by liver biopsy.2
CONCLUSION
With rising rates of obesity, NAFLD has become very common, affecting 20–30% of the general population.7,15,21 Although the majority of those with NAFLD have mild disease, a significant proportion have advanced liver disease and are at risk of liver-related complications. Clinicians should have a high index of suspicion for NAFLD and follow a diagnostic and staging pathway to identify those who need more specific treatment or surveillance for the associated complications. An example of such a pathway is shown in Figure 1; however, this pathway can be modified depending on the availability of tests locally.
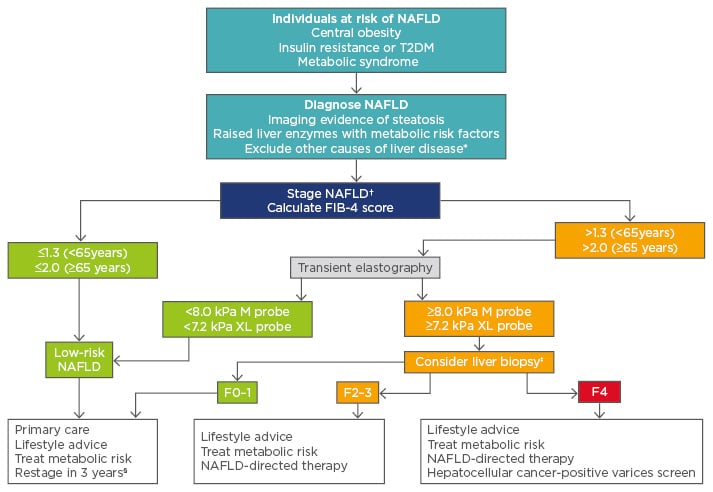
Figure 1: A potential algorithm for the diagnosis and staging of nonalcoholic fatty liver disease.
*Other causes include harmful alcohol consumption, hepatotoxic medications, viral hepatitis, autoimmune liver disease, haemochromatosis, coeliac disease, Wilson’s disease. †The FIB-4 score is unreliable in young patients (<35 years) so consider transient elastography first-line. ‡A liver biopsy is required to diagnose NASH if patients are to receive specific NASH treatment. §Restage with FIB-4 if previously low or transient elastography if FIB-4 was previously high.
FIB-4: Fibrosis-4 score; NAFLD: nonalcoholic fatty liver disease; NASH: nonalcoholic steatohepatitis; T2DM: Type 2 diabetes mellitus.








