Abstract
Clinical presentation of hepatocellular carcinoma (HCC) can vary from asymptomatic patients to patients presenting variable symptoms such as pain, lethargy, jaundice, hepatic encephalopathy, anasarca, ascites, variceal bleeding, diarrhoea, paraneoplastic symptoms, cutaneous manifestations, and abnormal laboratory values. Diagnosis of HCC is based on computed tomography (CT), magnetic resonance imaging (MRI), and tumour markers. The most commonly used is alpha fetoprotein.1,2 MRI is the imaging method of choice, although it has decreased sensitivity in detecting lesions <2 cm.3 Other possibilities include biomarkers such as embryonic antigen, protein antigen, enzymes and isoenzymes, cytokines, and genetic biomarkers. Liver biopsy is used in selected patients who do not present typical features of HCC on CT or MRI. Surveillance by ultrasound is recommended every 6 months in cirrhotic patients. The Barcelona Clinic Liver Cancer (BCLC) scoring system has been proposed for staging of HCC, and numerous scoring systems have been developed to evaluate progression and determine treatment possibilities; they take into account the clinical as well as the laboratory and pathological criteria, biomarkers, biopsy, and imaging methods.
CLINICAL PRESENTATION
Hepatocellular carcinoma (HCC) patients are frequently asymptomatic and the appearance of symptoms can signal the development of severe disease. Symptoms can appear early in patients with HCC due to chronic liver disease. In 90–95% of HCC patients a triad of right upper quadrant pain, palpable mass, and weight loss4 manifest; other symptoms that can be mentioned are jaundice, hepatic encephalopathy, anasarca, ascites, variceal bleeding, diarrhoea, paraneoplastic symptoms, cutaneous manifestations, and abnormal laboratory values.5-8 In non-cirrhotic patients, physical examination findings can be abdominal distention, anorexia, hepatomegaly, wasting, and right upper quadrant pain. A fatal complication of HCC is tumour rupture in which the patient experiences hypotension, irritation of the peritoneum, and severe abdominal pain.5 One of the paraneoplastic symptoms manifested in HCC is bone pain associated with hypercalcaemia, which is caused by osteolytic metastasis; other symptoms include erythrocytosis, hypoglycaemia, and androgen insensitivity syndrome.5,6 Erythrocytosis is due to an increase in the erythropoietin production in HCC patients.9 Overproduction of vasoactive intestinal peptide and gastrin are common causes of watery diarrhoea, mainly in cirrhotic patients due to their secreting characteristics.5-7 Cutaneous manifestations of HCC include pityriasis rotunda; Leser-Trélat sign, which is the appearance of multiple seborrheic keratoses; dermatomyositis; and pemphigus foliaceus. Porphyria cutanea tarda has been associated with HCC patients with chronic hepatitis C virus.5,8
TUMOUR MARKERS
Embryonic antigen, protein antigen, enzymes, isoenzymes, cytokines, and genetic biomarkers all have a potential utility in the detection of HCC.10
Embryonic Antigen
Alpha fetoprotein (AFP) is a glycoprotein that is normally produced during fetal life by the fetal liver and yolk sac. In adults, elevated AFP can indicate ongoing disease and patients with HCC often show elevation in serum concentration of AFP.10 Another cause of elevated AFP can be related to chronic hepatitis C virus infection.11 AFP has three glycoforms: AFP-L3 is the main isoform of AFP in the serum of HCC patients and can be detected in approximately one-third of patients with small HCC (<3 cm) when cut-off values of 10–15% are used.2,12 This biomarker was found to be related to recurrence rate when it increases >10% or rises after normalisation with treatment. AFP-L3 was found to be associated with poorly differentiated and advanced HCC. However, when comparing between total AFP level and AFP-L3 it seems that the latter is more specific in diagnosing HCC.2,12 It has been suggested that AFP-L3 can be used as a screening tool because it can appear up to 12 months before imaging methods detect HCC, but meta-analyses concluded that, due to poor sensitivity, it has limited function in screening studies.2,13 Clinical studies showed that at a cut-off of 20 ng/mL, the sensitivity of serum AFP is 41–65% and the specificity is 80–94%. However, there are several disadvantages of AFP in regard to early diagnosis. Firstly, the positive rate of AFP in HCC is about 60–80%, which is not enough to make it a sensitive biomarker. Secondly, false positive results can be recorded during pregnancy and when the patient also has liver or gastrointestinal diseases. Thirdly, false negative results can occur.10 Although the serum biomarkers discussed next may show similar or better sensitivity and specificity, AFP is still the most commonly used screening biomarker.
Protein Antigen
Heat shock protein (HSP) is a molecule that forms in cells exposed to stress, including carcinogenesis. These proteins protect the cells and promote the cells to repair the damage from the anxious stimuli. HSP 70 and HSP 27 have been found in HCC tissues. HSP 70 correlates with portal vein invasion and tumour stage and size. HSP 27 has been seen in hepatitis B virus-infected patients with HCC. A previous study has shown that the sensitivity of HSP 70 is 57.5% and the specificity is 85%.10,14
Glypican-3 is a family of the heparan sulfate proteoglycans linked to the cell membrane by a glycosylphosphatidylinositol linkage.15 Glypican-3 plays a role in cell growth regulation, migration, differentiation, and development. Levels of glypican-3 were increased in HCC patients, and no correlation to tumour size, stage, or AFP was indicated. A sensitivity of 77% and specificity of 96% was correlated to this marker. Squamous cell carcinoma antigen (SCCA) is a serine protease inhibitor that can be found in high levels in HCC patients and helps tumour cells evade apoptosis.10,16 High levels of SCCA can indicate the early stages of HCC. Specificity and sensitivity are 46% and 84%, respectively; this finding makes SCCA a complementary marker for AFP.10
Golgi protein 73 and tumour-associated glycoprotein were elevated in patients with HCC and can be used as markers for detection of HCC.10
Enzymes and Isoenzymes
Des-γ-carboxyprothrombin (DCP), γ-glutamyl transferase (GGT), and α-1-fucosidase (AFU) are supplementary markers to AFP in the detection of HCC.10 DCP, also known as PIVKA-II, occurs due to the absence of vitamin K. It has better sensitivity for HCC lesions >5 cm in diameter. When combined with AFP, it gives a better prediction of HCC recurrence 6 months post-surgery. DCP enables better prediction of larger tumour and vascular invasion.10 GGT has low serum levels in healthy adults; however, in patients with liver disease such as cholestasis, inflammation, or benign or malignant tumour, the level of GGT increases. The enzyme has relatively low sensitivity (43%) and can be used as a supplementary marker together with AFP.10
Alkaline phosphatase is a liver enzyme that increases in cases of several liver diseases and is known to play a role in the diagnosis and screening of HCC. It may also predict survival, with a cut-off >121 U/L, a sensitivity of 41.4%, and a specificity of 85.9%.17 AFU is a lysosomal enzyme. It can be found in healthy adults and displays elevated levels in patients with HCC. When used alone, AFU has poor specificity; however, when combined with AFP it can greatly improve AFP sensitivity and specificity.10
Cytokines
Transforming growth factor-β1 has been indicated to be a good supplementary marker in the diagnosis of HCC as it has higher sensitivity than AFP. Transforming growth factor-β1 has a role in cell regulation, including angiogenesis, differentiation, invasion, and cell proliferation.10,18 It has an immunosuppressive effect by inhibiting the proliferation of natural killer cells and cytotoxic T cells, which allows tumour cells to grow.10 Vascular endothelial growth factor functions in endothelial migration, new vessel formation, invasion, and metastasis. The expression of vascular endothelial growth factor in HCC patients is upregulated and correlates to prognosis and recurrence of the tumour.10 Interleukin (IL)-8 is a chemokine that has prognostic and diagnostic properties in HCC patients. IL-8 plays a role in chemotaxis, enzyme release, and expression of adhesion molecules in neutrophils as well as tumour proliferation and metastasis. When compared to healthy adults the levels of IL-8 in HCC patients were higher. IL-8 overexpression has been shown to be an indicator of tumour size, absence of tumour capsules, and invasion of the veins.18
Genetic Biomarkers
microRNAs (miRNAs) are new biomarkers for HCC diagnosis. miRNAs are small non-coding RNAs that effectively block translation by promoting the degradation of target mRNAs or binding to complementary sequences in the 3’ untranslated region. miR-29, miR-199a/b-3p, and miR-122 were downregulated in HCC cells. miR-21 was the only one that was upregulated in the HCC cells. A sensitivity of 87.3% and specificity of 92% were shown for miR-21.10
IMAGING METHODS
Ultrasound
Ultrasound (US) is a widely available non-invasive technique. It is free of radiation and has an important role in the surveillance of HCC in cirrhotic patients.3,5 Some authors recommend US modality in combination with AFP in biannual screening of high-risk patients in order to reduce HCC mortality.19 Nevertheless it has disadvantages, especially the difficulty in differentiating between benign and malignant tumours. When US is combined with AFP it aids in the diagnosis of HCC.3
HCC lesions <3 cm are usually hypoechoic relative to the surrounding tissues while lesions >3 cm are hyperechoic with a mosaic or infiltrative pattern and may have a thin capsule.3 Non-contrast-enhanced US is not the method of choice in detecting HCC, and it has been replaced with computed tomography (CT) and magnetic resonance imaging (MRI) due to their higher sensitivity and positive predicting value.3
HCC lesions are characterised by high vascularity, and the contrast-enhanced US can display the intrahepatic vascular flow in HCC lesions and help in the diagnosis. In addition, contrast-enhanced US can differentiate between bland thrombus and tumour invasion.5 Contrast-enhanced US has a higher accuracy of biopsy, which lowers the false negative rate of malignant lesions. Disadvantages of US include association with obesity and the requirement for necessary operator experience. Moreover, US has difficulties in differentiating between benign and malignant lesions in the context of nodular cirrhosis.3
Computed Tomography
CT is a widely used radiological technique for diagnosis of HCC. The most commonly used type of CT is the multidetector CT, which has a higher quality, thin sections, and three-dimensional capabilities but is not contrast-enhanced. Intravenous contrast is used for better diagnostic results and can be divided into an arterial phase, a portal phase, and a delayed phase.3,5 In the development of HCC, which is a hypervascular lesion, there is loss of portal venous blood supply and formation of collateral arterialisation. When contrast material is introduced intravenously there is an increase in arterial enhancement followed by a delay in the venous ‘washout’ phase. This pattern is a characteristic sign for diagnosis of HCC (Figure 1).21 CT showed better sensitivity than US but poorer sensitivity than MRI.22
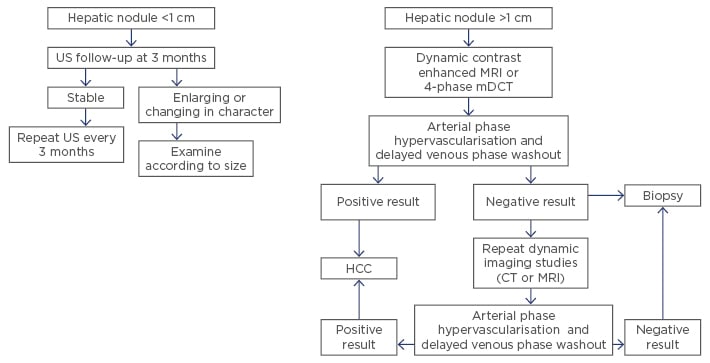
Figure 1: American Association for the Study of Liver Disease (AASLD) practice guidelines for hepatocellular carcinoma surveillance and diagnosis.20
US: ultrasound; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; MDCT: multidetector computed tomography; CT: computed tomography.
Magnetic Resonance Imaging
MRI has properties similar to a CT scan and it is free of radiation but is an expensive imaging method. In contrast-enhanced T1 W with gadolinium, images in the hepatic artery, portal vein, and delayed phase show improved detection and characterisation of small HCC lesions and are superior to multiphasic helical CT. Combined double contrast MRI, which includes gadolinium together with super paramagnetic iron oxide, is found to be highly sensitive (92%) in the detection of HCC >1 cm in size and better than either of them alone. T2 weighted images show hypo-intensity due to the uptake of super paramagnetic iron oxide or ferumoxide particles taken up by Kupffer cells.23 MRI sensitivity is high; however, it causes difficulties in evaluating tumours <2 cm. Nevertheless, it is the imaging method of choice for HCC.3,5
Nuclear imaging positron emission tomography (PET) has limited use as a diagnostic tool in HCC. It is instead used to evaluate the spread of HCC to tissues outside the liver. PET imaging is based on radiolabelled glucose (fludeoxyglucose [18F-FDG]), which binds into neoplastic cells demonstrating increased metabolic activity. HCC tumours with good or moderate differentiation and metastasis properties may not produce a high level of metabolism requirements as compared to that of neighbouring tissues. 18F-FDG was first used in the diagnosis of HCC, but due to high expression of glucose-6-phosphatase in HCC cells this imaging was not sensitive enough. However, when 11C-acetate was introduced, the sensitivity and specificity were increased, but it still could not distinguish between HCC and benign lesions. Recent studies of dynamic PET with kinetic modelling show that the differentiation of HCC from benign liver tumours is possible.3,5
Liver Biopsy
Confirmation of HCC can be done by means of percutaneous fine needle aspiration biopsy or core biopsy, guided by US, CT, or transjugular biopsy. It has a higher sensitivity and specificity than non-invasive techniques and can be used to detect HCC with characteristics that do not meet the radiological or laboratory parameters of HCC.5,24 Complications are not common during biopsy but some can be seen such as infections or haemorrhage and the risk of tumour spread from the biopsy needle. Limitations for biopsy are platelet count <50,000 mm3, or international normalized ratio (INR) >2. Transjugular biopsy overcomes these problems but results in non-targeted biopsy and therefore is usually appropriate for diffuse liver processes.25 Mortality rates for biopsy procedures are low and range between 0.006% and 0.3%, although sampling errors and repeated biopsy are frequent clinical issues.5,26
Surveillance
Surveillance of HCC has an important role in the detection of early tumours and treatment, thus it can lead to more effective treatment. AFP, US, CT, and MRI are examples of methods in which surveillance can be carried out.19,27-30 According to the international guidelines of the American Association for the Study of Liver Disease (AASLD), screening of patients with cirrhosis and patients at high risk of HCC is mandatory, and US should be used every 6 months. AFP, which is the most widely used biomarker, has not been proven to be adequate for surveillance even when combined with US.
Nevertheless, when combining AFP together with AFP-L3 and DCP, as in the GALAD model, the levels of sensitivity range between 79.3% and 99.2% with a specificity of 50–88.3%.31 In Japan, the Japanese Society of Hypertension (JSH) recommendations for patients at extremely high risk of HCC (cirrhosis, hepatitis B virus) are to have US examination every 3–4 months, together with measurement of AFP, AFP-L3, DCP every 3–4 months.1 The contrast-enhanced CT scan and MRI are the most common diagnostic tools in HCC detection. There is much debate regarding the cost-effectiveness of HCC surveillance;32 however, US every 6 months in cirrhotic patients is the recommended and most cost-effective method for surveillance.21,27
STAGING
Barcelona Clinic Liver Cancer
Currently, the only validated staging is the Barcelona Clinic Liver Cancer (BCLC) staging, and it can be divided into five categories: very early, early, intermediate, advanced, and terminal. The American Association for the Study of Liver Disease (AASLD) recommends BCLC staging for HCC (Table 1). This staging helps to predict the outcome and the appropriate treatment (Table 2).34
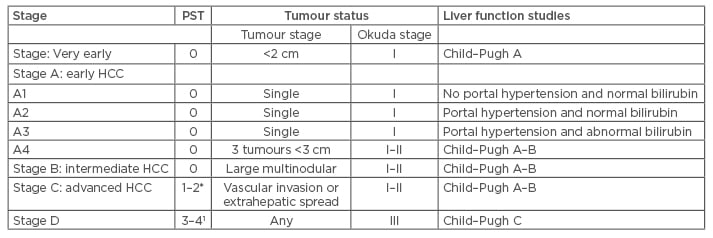
Table 1: Barcelona Clinic Liver Cancer staging classification.34,35
Stage A and B, all criteria should be fullfilled; *: Stage C, at least one criteria should be fulfilled; PST 1–2 or vascular invasion/extrahepatic spread; 1: Stage D, one or more criteria should be fulfilled; PST 3-4 or Okuda stage III/Child–Pugh C. Small HCC <5 cm, large HCC >5 cm.36 PST: performance status test; HCC: hepatocellular carcinoma.
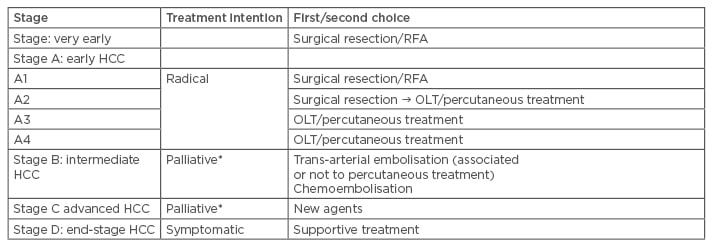
Table 2: Treatment schedule proposed for hepatocellular carcinoma cirrhotic patients according to the Barcelona Clinic Liver Cancer classification system.34,37
*In the setting of Phase II investigations or randomised control trials.
HCC: hepatocellular carcinoma; RFA: radiofrequency ablation; OLT: orthotopic liver transplantation.
Okuda Score
The Okuda score is a prognostic score that includes tumour size higher or lower than 50% of the liver, the presence of hepatic failure markers and factors such as presence or absence of ascites, and serum albumin and bilirubin levels. The score contributes to the diagnosis of HCC and can be divided into three stages. The survival rate of Stage I patients is 11.5 months, Stage II patients 3 months, and Stage III patients 0.9 months.34
SCORES
Several different scores were dedicated to the evaluation of the progression of HCC. The Child– Turcotte–Pugh (CTP) classification, Model of End Stage Liver Disease (MELD), Okuda score, and Cancer of the Liver Italian Program (CLIP) score are all prognostic factors for the determination of survival and treatment of HCC patients.
Child–Turcotte–Pugh System
The primary Child–Turcotte system included clinical parameters of ascites, encephalopathy, bilirubin levels, albumin levels, and nutritional status. In 1973, Pugh modified the nutritional status for prothrombin time (Table 3).16 Although it has no HCC specific parameters, the CTP system is instrumental in grading liver function and is part of HCC evaluation. It also helps determine the proper treatment where surgery has the highest cure potential. The CTP system has some disadvantages, which include day-to-day fluctuation in key parameters and inter-laboratory variations. Nevertheless, the CTP score has been incorporated into other HCC-specific staging systems such as BCLC.34
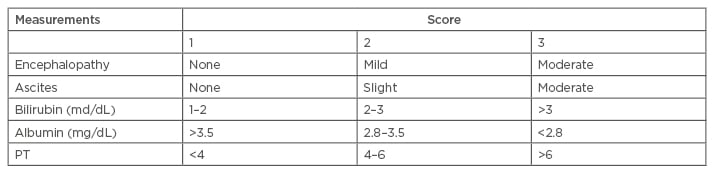
Table 3: Child–Turcotte–Pugh score.34
Stage A: 5–6 points; Stage B: 7–9 points; Stage C: 10–15 points.
PT: prothrombin time (seconds prolonged).
Model of End Stage Liver Disease
The MELD was originally developed for predicting survival of patients following transjugular intrahepatic shunt.38 The model is a logarithmic score composed of serum creatinine, total serum bilirubin, and INR. Patients with HCC may be prioritised for curative orthotopic liver transplantation upon this scoring, but, while waiting for MELD score to move them up on the graft allocation priority list, a worsening in the condition of patients in early stage HCC may occur. This problem has been solved by giving ‘extra’ points to HCC patients.34
MELD scoring system
MELD Score = 9.57 x ln (serum creatinine in mg/dL)+ 3.78 x ln (serum bilirubin in mg/dL) + 11.2 x ln (INR) + 6.43.34
Cancer of the Liver Italian Program Score
The CLIP score is the most recently developed prognostic scoring system for HCC. It combines tumour stage and macroscopic tumour morphology, serum AFP levels, and the presence or absence of portal vein thrombosis with an index of the severity of cirrhosis to determine a prognostic score range 0–6 (Table 4). The score has several disadvantages. Firstly, it can discriminate between patients with a 0–3 score but cannot discriminate between patients with a score range of 4–6.
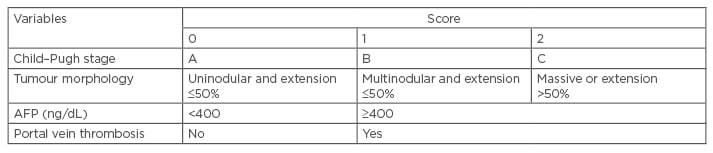
Table 4: Cancer of the Liver Italian Program scoring system.34
AFP: alpha fetoprotein.
Secondly, the system is not able to select the group that will benefit from curative and aggressive treatment. Thirdly, the stratification ability of this score is poor and approximately 80% will show a score of 0–2.34,39
Chinese University Prognostic Index
The Chinese University Prognostic Index (CUPI) is another method by which HCC risk can be measured. A Cox regression model that determines the relationship between survival and several variables has been included in this index. The relation between tumour, node and metastasis (TNM) staging and 18 other clinical factors has been studied, and the outcome was a survival rate of 3 months. The model has confirmed TNM staging as a highly valuable predictor of 3-month survival. The model also identified presentation of asymptomatic disease, AFP level, total bilirubin, serum alkaline phosphatase, and clinical detection of ascites as significant prognostic factors. CUPI and CLIP score were the best models in prediction of survival in advanced HCC patients among 12 different systems analysed.38,40







