Abstract
Transjugular intrahepatic portosystemic shunt (TIPS) offers an effective treatment for patients with complications of portal hypertension, specifically prevention of variceal rebleeding and recurrent or refractory ascites. TIPS reduces portal pressure and increases effective blood volume and cardiac output, but long-term adverse effects may include increased risk of liver failure, hepatic encephalopathy, and cardiac dysfunction. As such, TIPS is not indicated for primary prophylaxis of variceal bleeding. Critical to the success of TIPS is a dedicated, multidisciplinary team, along with careful patient selection and appropriate timing of the procedure; for example, in high-risk patients TIPS may offer clinical benefits when performed early in the disease course. Important patient factors to consider before performing TIPS include cardiac and renal function, severity of liver dysfunction, history of hepatic encephalopathy, and inflammatory status. Recent studies indicate that technical considerations, specifically diameter control and downsizing covered stents, may reduce adverse events and increase clinical benefits of TIPS. This review focusses on the optimisation of the use of a covered TIPS endoprosthesis in patients with portal hypertension-related complications, with consideration of evolving practices, patient selection, and multidisciplinary co-operation. Further research and patient stratification are necessary to enhance understanding of the optimal use of covered TIPS and to ensure that the right patients receive TIPS at the right time.
INTRODUCTION
Portal hypertension is the main complication of cirrhosis and occurs due to increased pressure within the portal vein(s).1 Clinically significant portal hypertension is defined as a hepatic venous pressure gradient (HVPG) >10 mmHg.2 Elevated portal pressure increases cardiac output and reduces systemic vascular resistance, increasing blood flow and leading to serious complications.1 These sequelae, variceal bleeding and refractory ascites, are associated with poor patient prognosis.
Treatment of portal hypertension focusses on preventing or managing complications and, at first-line, is dependent largely on pharmacological approaches, which include non-selective beta-blockers (NSBB). NSBB reduce cardiac output and induce splanchnic vasoconstriction, efficiently treating portal hypertension. However, there is a significant degree of treatment failure when NSBB are indicated for either primary or secondary prophylaxis of variceal bleeding.3,4 In addition to NSBB, endoscopic band ligation is also appropriate for secondary prophylaxis;2 however, this therapy alone is associated with high rates of rebleeding compared with its use in combination with NSBB.5
The predominant treatments for ascites are diuretics or large-volume paracentesis (LVP) and albumin infusion. Diuretics are the first-line treatment and act to improve sodium balance and circulation;6 however, refractory ascites, defined as ascites that cannot be mobilised or with early recurrence that cannot be controlled with medical treatment,7 develop in >10% of patients after initial ascites presentation.8 There are two causes of refractory ascites: diuretic-intractable or diuretic-resistant.8 LVP is mandatory if first-line therapy fails or for tense ascites;6 however, it is associated with recurrence rates as high as 89%.9 Consequently, LVP is frequently repeated, which places a high burden on patients and the healthcare system.6
For patients with variceal bleeding or ascites who do not respond to first-line treatment, transjugular intrahepatic portosystemic shunt (TIPS) is a well-established procedure. This review discusses optimisation of patient care and safety of covered TIPS in patients with portal hypertension, focussing on recent technical developments and considerations for patient selection.
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT FOR THE MANAGEMENT OF COMPLICATIONS RELATED TO PORTAL HYPERTENSION
TIPS is a percutaneously created connection between the portal and hepatic veins, which diverts blood flow to reduce pressure between the portal and systemic circulations.10 Pioneered in 1969,11 the TIPS procedure has gained acceptance worldwide and is indicated for the treatment of portal hypertension complications. TIPS increases effective blood volume, an effect that is desirable, particularly in patients with refractory ascites and impaired renal function.12 Clinical contraindications for the placement of TIPS include severe liver and renal failure, heart failure, severe portopulmonary hypertension, recurrent or persistent overt hepatic encephalopathy (HE), and uncontrolled sepsis.13
Various scoring systems are used to stratify patients based on risk and predict post-TIPS survival. The model for end-stage liver disease (MELD) and Child–Pugh scores (CPS) were developed to predict mortality after shunting and incorporate parameters including creatinine, bilirubin, and albumin.14,15 The Baveno VI consensus considers CPS Class B patients with active bleeding at presentation or CPS Class C (<14) patients as high-risk and indicated for TIPS;2 however, the recent European Association for the Study of the Liver (EASL) guidelines indicate that high-risk patient criteria require further study.16 Furthermore, a recent study has shown that CPS Class B patients who receive standard therapy, with or without active bleeding, show reduced mortality in comparison with CPS Class C patients, suggesting TIPS may not be necessary for Class B patients.17 MELD cut-off scores, ranging between 10 and 19, are adopted for TIPS, with a high score indicative of a reduced chance of survival;9,13 although, one study found mortality is lower following TIPS compared with LVP across a series of MELD scores, including <10 and ≥19.9 Recently, a large Chinese study reported that TIPS improves short and long-term survival, particularly in patients with a high MELD score (≥19).18 Liver failure is reported in almost 10% of patients with a MELD score <12,19 indicating that there is no ideal cut-off score and that MELD may not be sufficient for excluding liver failure post-TIPS. Other factors, such as bilirubin levels <3 mg/dL and platelet count >75×109/L, may be superior indicators of survival and useful for patient selection.20
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT STENT CHARACTERISTICS
The aim of TIPS is to reduce portal pressure enough to treat existing, and prevent further, complications of portal hypertension, but to avoid complications due to overshunting, such as liver dysfunction and HE. Bare stents have previously been used for TIPS but now polytetrafluorothylene-covered stent grafts are recommended, which reduce the risk of TIPS dysfunction and improve shunt patency when compared with bare stents.16,21
Stent diameter is one of the parameters that can be used to control shunting: the wider the shunt diameter, the greater the degree of shunting. While an optimal HVPG post-TIPS is considered <12 mmHg for variceal bleeding, a threshold for refractory ascites is less well-defined21 and low HVPG (<8 mmHg) may be associated with increased mortality and risk of complications.22,23 Evidence suggests 8 mm stents are effective in reducing portal hypertension compared with medical treatment.4 While one study of 45 cirrhotic patients found 10 mm stents to be superior to the 8 mm stent, this study was terminated prematurely due to reported side effects.24 A larger study in 127 patients found there was a significant reduction in the risk of HE and liver failure with 8 mm stents compared with 10 mm stents.25 In addition, a recent study demonstrated underdilation of covered stents (<8 mm) reduces HE in comparison with stents ≥8 mm; however, this study included a heterogeneous patient group.23 Importantly, underdilated covered stents have been shown to passively increase in diameter over time.26,27 The extent of expansion can vary, with reports ranging from expansion limited to <1 mm in diameter23 to expansion to nominal diameter.26 Recent research has indicated that the use of a novel diameter-controlled expansion stent maintains its diameter and improves shunt patency and MELD-serum sodium-based scores, as well as reducing the number of hospital readmissions post-TIPS, specifically for sepsis and ascites.28
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT TEAMWORK
For effective TIPS delivery, a dedicated, multidisciplinary team is required, ideally consisting of a hepatologist, radiologist, and a transplant surgeon.13 The importance of experience was highlighted in a recent epidemiological study examining potential causes of post-TIPS mortality.29 This study found that mortality rates were associated with the volume of TIPS procedures performed, with performance of ≥20 TIPS per year reducing the risk of complications.29 The Baveno VI consensus states that high-risk patients with variceal bleeding should be referred for TIPS within 72 hours of initial pharmacological/endoscopic treatment or when alternative treatments fail to manage the complications.2 Despite these recommendations, a recent multicentre audit in France reported that only 7% of 326 eligible patients had access to TIPS.30 Sarwar et al.29 also reported that expert centres carrying out ≥20 TIPS per year accounted for only 38% of all TIPS procedures,29 highlighting that both accessibility and experience may be limited. Lack of availability of TIPS at centres means that timely implementation may be restricted; however, patient referral to a centre experienced in performing TIPS within a relatively short distance may help overcome this.30
CLINICAL EFFECTS OF TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT IN PATIENTS WITH VARICEAL BLEEDING
When indicated for variceal bleeding, TIPS should be considered within three settings: as a rescue treatment, following failure of secondary prophylaxis, or as a pre-emptive treatment (early TIPS).
Rescue Transjugular Intrahepatic Portosystemic Shunt
Rescue or salvage TIPS refers to the treatment of uncontrolled or recurrent bleeding. Following first-line treatments, approximately 20% of patients with varices experience rebleeding.31 Although numerous studies have demonstrated that TIPS is more effective than NSBB and/or endoscopic treatment, previous studies have shown no improvement in overall survival or variable safety outcomes with TIPS versus first-line treatments.4,32-34 Such findings, along with the increased cost of TIPS compared with pharmacological treatment,33 have led to the use of TIPS as a rescue therapy only in cases of persistent or rebleeding within 5 days of primary prophylaxis.2,13,16,21
Failure of Secondary Prophylaxis
In the secondary prophylaxis setting, TIPS is only indicated following failure of appropriate secondary prophylaxis: combined NSBB and endoscopic treatments.13,16 TIPS may still be beneficial for secondary prophylaxis since studies have indicated that, despite no change in mortality, TIPS is superior to endoscopic and pharmacological treatment following failure of primary prophylaxis.4,32
Early Transjugular Intrahepatic Portosystemic Shunt
A subset of patients show a survival benefit with early TIPS. Early or pre-emptive TIPS is conducted after acute bleeding in patients with a high risk of rebleeding or treatment failure.2 In these patients, including those with HVPG ≥20 mmHg,35 TIPS performed within 24–72 hours of diagnosis has shown improved survival rates compared with pharmacological and endoscopic treatments.35-39 These studies are supported by recent meta-analyses examining the effects of early TIPS compared with first-line treatments, which showed improved mortality and rebleeding risks.40,41
Reported differences in survival may be due to selection criteria. Indeed, studies conducted in a heterogeneous patient group have shown no survival advantage,32 while studies considered to have a focussed patient population have demonstrated survival benefit.36,37 One study comparing early TIPS with medical and endoscopic treatment demonstrated an elevated risk of cardiac failure, as well as no survival benefit with TIPS.39 Again, these findings may be due to patient selection, which included a large number of CPS Class C (14–15) patients and patients with prior cardiovascular problems. This suggests that some patients may be too high-risk to benefit from TIPS. In addition to patient selection, differences in survival may be due to the stents used. García-Pagán et al.36 found covered stents reduced recurrent bleeding, and the small amount of rebleeding reported was mainly observed when bare stents were used. However, new data confirm the benefit to survival from early TIPS.18,42
CLINICAL EFFECTS OF TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT IN PATIENTS WITH ASCITES
TIPS mobilises ascites, showing particular benefits in patients with refractory ascites.43 Although TIPS is more effective than LVP,44-46 it is only indicated for patients unresponsive to repeated (>3) LVP.6,16,21 This recommendation is based on studies and meta-analyses that have shown efficacy of TIPS but variable impact on survival and adverse events.9,45-47 In addition, patients with refractory ascites are often not eligible for TIPS based on contraindications and exclusion criteria.16
As with rebleeding, management of ascites is based on individual patient characteristics. Patients with a high frequency of LVP treatments should be considered for TIPS; LVP improves symptoms short-term but has a negative impact on systemic haemodynamics and renal function, which may be overcome by TIPS.6 Patients with diuretic-induced renal dysfunction and those that are unlikely to respond to diuretics should also be considered.48 In a French multicentre study, patients who had not been managed long-term with LVP showed superior survival benefit with TIPS, as well as no increase in HE.44 In this specific study, the majority of patients had no previous history of HE, were CPS Class B, and aged <65 years.44
Patients with later-stage ascites tend to rapidly deteriorate because refractory ascites can be accompanied by cardiac problems, hepatic dysfunction, and hepatorenal syndrome (HRS). Approximately 50% of patients with refractory ascites develop HRS and survival probability is dependent on age, type of HRS, and CPS class; the mean survival after developing Type 1 HRS is 1 week, while the probability of survival with Type 2 HRS is 38.5% at 1 year.8 TIPS may therefore improve survival and limit further complications; the optimal window of opportunity to intervene should be early in the disease course, as soon as ascites are classed as diuretic-intractable or refractory. In addition to timing, smaller diameter stents are recommended for patients with refractory ascites16 and, as such, the use of diameter-controlled expansion stents may benefit these patients further.28
TIPS has shown superior transplant-free survival compared with LVP and albumin treatment43 (Figure 1), and improves kidney function in patients with renal impairment.12,48 It should be noted that TIPS may also reduce glomerular filtration rate (GFR); Allegretti et al.48 reported that in 138 patients who received TIPS, estimated GFR declined in 26% of cases. Renal failure can be an associated complication of TIPS.12 A retrospective study showed that TIPS improved survival in comparison with paracentesis, yet the improved survival benefit declined over time,49 indicating TIPS may provide a bridge to liver transplantation. Organ allocation is based on MELD scores50 and parameters contributing to this score, such as creatinine, are affected post-TIPS. Consequently, the impact of TIPS placement in patients awaiting a liver transplant should be considered carefully. Currently, it is recommended that eligible patients are transferred to a specialist centre and a TIPS procedure be discussed with an expert team.13
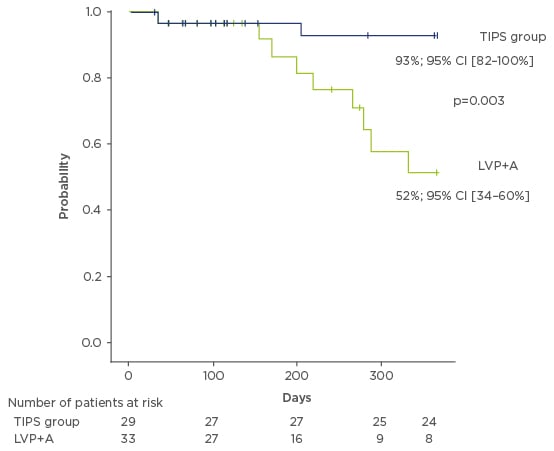
Figure 1: Probability of transplantation-free survival in patients with transjugular intrahepatic portosystemic shunt versus large-volume paracentesis and albumin infusion.44
A: albumin; CI: confidence interval; LVP: large-volume paracentesis; TIPS: transjugular intrahepatic portosystemic shunt.
PATIENT CARE AND TIMING OF TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
TIPS shows clear benefits for controlling variceal bleeding and eradicating refractory ascites, yet timing and patient selection are crucial for the success of this procedure. Many factors contribute to early mortality rates post-TIPS, including pre-TIPS severity of liver dysfunction, renal failure, cardiac function, previous history of HE, and inflammatory status. Assessment of these factors should be made pre-TIPS to improve outcomes.
Liver dysfunction post-TIPS, thought to occur through excessive shunting and reduced portal venous perfusion, may contribute to the pathophysiology of HE, caused by hepatic insufficiency and increased portosystemic shunting.51 However, controlled studies using covered stents have not shown an increased incidence of HE post-TIPS,32,36,44 and HE symptoms can be alleviated upon embolisation of any spontaneous physiological shunts present.52 To limit liver dysfunction, bilirubin levels and platelet count should be examined.20 Detrimental changes in cardiac output will influence outcomes, therefore the EASL guidelines state that functional cardiac testing should be part of a pre-TIPS assessment, as well as a standardised assessment of systolic and diastolic function for patients with decompensated cirrhosis.16 As with many chronic conditions, systemic inflammation is present in cirrhosis and high levels of proinflammatory markers in the hepatic vein are reportedly associated with poor outcomes and organ failure;53 the inflammatory profile of patients could therefore also be examined prior to TIPS.
Regardless of the aforementioned factors, timing is crucial, as highlighted in the early TIPS setting.36,38 This is supported by the Baveno VI consensus,2 although physician adherence to these guidelines and availability of TIPS are lacking.30 In addition, no pre-TIPS testing and patient stratification are necessary in the context of rescue TIPS because in this instance TIPS provides a last resort.
THE FUTURE OF TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT THERAPY
In order to maximise the benefit of TIPS in the right patients at the right time, the previously discussed aspects and limitations need to be considered. Further studies are required to confirm the role of timing and patient selection, particularly for patients with refractory ascites. Currently, during the preparatory phase pre-TIPS, patients should be routinely assessed for liver and cardiac function. Further testing may also be beneficial, including renal function assessment and a measurement of liver stiffness, to aid in the prediction of liver failure post-TIPS. Numerous testing strategies are also available for HE,51 including a critical flicker frequency test that has been reported to predict initial episodes of HE in cirrhotic patients.54,55 More data are required to assess the role of this test in the detection of post-TIPS HE and to determine whether this could provide a screening tool. More thorough routine testing prior to and during follow-up of TIPS may reduce the rates of adverse events and further complications.
Further to its use for ascites and variceal bleeding, TIPS has potential in other indications, including portal vein thrombosis, HRS, hepatic hydrothorax, Budd–Chiari syndrome, sinusoidal obstruction syndrome, rare bleeding (e.g., ectopic varices and portal hypertensive gastropathy), and variceal embolisation.10 Portal vein thrombosis in particular is a frequent occurrence in cirrhotic patients and TIPS has been shown to reduce its progression and further complications,56 although data exploring it as an indication are limited. TIPS can also reduce malnutrition through a positive effect on energy balance, increasing body weight, muscle mass, energy expenditure, and energy intake (Figure 2).57 Furthermore, reduced muscle mass may represent a risk factor for HE development post-TIPS and, as such, although sarcopenia is not an indication for TIPS, it may be improved in some patients and impact outcomes post-TIPS.58-60
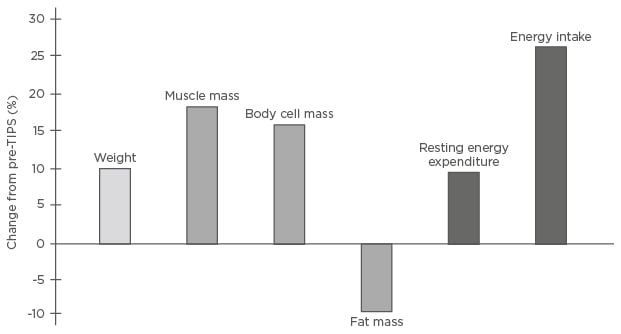
Figure 2: Effects of transjugular intrahepatic portosystemic shunt on energy balance.57
Percentage changes from baseline in 21 patients with liver cirrhosis 6 (5.0–7.4) months after TIPS insertion.
TIPS: transjugular intrahepatic portosystemic shunt.
In patients who do not fit the ideal criteria (Table 1),61 TIPS may still provide the only viable treatment option. In such cases, the benefits and risks of TIPS must be balanced; when it provides the only treatment option or when transplantation is an option, more risks can be taken. However, when transplantation is not an option, or in the early TIPS setting, more caution is required. For these patients, optimising technical parameters, such as the use of novel diameter-controlled expansion stents, may provide a means of reducing adverse events by allowing physicians to better control diameter and target the optimal pressure gradient for a specific patient. This technology offers a promising solution for patients.
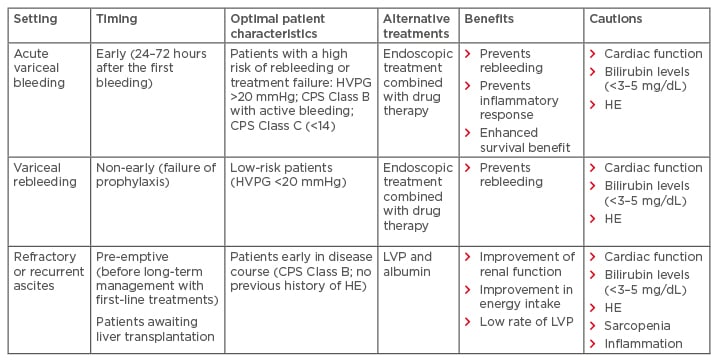
Table 1: Key patient groups with complications of portal hypertension that may benefit from transjugular intrahepatic portosystemic shunt.
CPS: Child–Pugh score; HE: hepatic encephalopathy; HVPG: hepatic venous pressure gradient; LVP: large- volume paracentesis.
Adapted from Trebicka.61
CONCLUSION
Covered TIPS shows clear clinical benefits, including reduced complications and improved transplant-free survival in a subset of patients with variceal bleeding and/or recurrent and refractory ascites. Importantly, patient selection should be considered carefully. Patients without a previous history of cardiovascular problems, liver dysfunction, and/or HE are prime candidates for TIPS, whereas patients with poor health, a high MELD score and CPS, high bilirubin levels and platelet count, poor cardiac reserve, and risk of HE should only be considered when all other options are exhausted and transplantation is the only route. Specialised TIPS teams are required to carry out pre-TIPS screening of such factors, as well as follow-up monitoring to improve patient outcomes. In addition, evolving TIPS practices, which show adoption of smaller diameter covered stents as well as novel diameter-controlled expansion TIPS, may offer an enhanced survival benefit, with data suggesting improved control of variceal bleeding, eradication of refractory ascites, reduced risk of complications, and improved nutritional status.
Further stratification of patients suitable for early TIPS, following a first variceal bleed or refractory ascites, will increase understanding of the ideal moment to offer TIPS in the treatment algorithm. Coupled with an increased number of, and referrals to, centres offering TIPS expertise, this will help ensure the right patients receive optimal care at the right time.








