BACKGROUND AND AIMS
Among patients with chronic liver disease (CLD), thrombocytopenia (TCP) is a frequent complication.1,2 Before invasive procedures and surgery in CLD patients with TCP, platelet transfusions (PT) have been used to increase platelet counts, but their use is limited by several factors, including the short lifespan of transfused platelets, alloimmunisation, and various haemolytic, allergic, and other secondary reactions, which can lead to hospitalisations.3
Lusutrombopag is an oral thrombopoietin receptor agonist that has been approved in Japan (2015) and the USA (2018) for treatment of TCP, and in Europe for severe TCP (2019), and is associated with CLD in patients undergoing a planned invasive procedure.4-6 In L-PLUS 1 (Japan) and L-PLUS 2 (global), two similarly designed, Phase III, multicentre, randomised, double-blind, placebo-controlled studies, patients with CLD and platelet count <50×109/L scheduled for an invasive procedure were randomised 1:1 to lusutrombopag 3 mg or placebo and dosed orally once daily for up to 7 days. Results showed that a higher proportion of lusutrombopag-treated than placebo-treated patients did not require a PT.3,7
Certain CLD aetiologies, such as alcohol abuse and hepatitis C virus, may cause bone marrow suppression and low thrombopoietin production.8 Accordingly, the authors undertook the pooled analysis described herein to evaluate the efficacy of lusutrombopag in patients with CLD by underlying disease aetiology.
METHODS
For the current analysis, data from the L-PLUS 1 and L-PLUS 2 per-protocol (PP) patient populations (defined as all randomised patients with no major protocol violations) were pooled and assessed by various underlying CLD disease aetiologies. The primary efficacy endpoint was the proportion of patients who required no PT prior to the invasive procedure and no rescue therapy for bleeding from randomisation throughout 7 days after the procedure. Treatment-emergent adverse events (TEAE) were also assessed by disease aetiology subgroup.
RESULTS
Of the 312 patients randomised, 270 were in the PP population (lusutrombopag: n=137; placebo: n=133). Underlying CLD aetiologies were present in the PP population in the following proportions: chronic hepatitis B: 10.7% (29/270); chronic hepatitis C: 47.8% (129/270); alcoholic hepatitis: 11.5% (31/270); nonalcoholic steatohepatitis: 8.5% (23/270); autoimmune hepatitis: 3.0% (8/270); and other (including multiple aetiology): 18.5% (50/270). The underlying aetiologies for CLD were generally similar between the two treatment arms. Overall, 73.7% (101/137) of lusutrombopag patients met the primary endpoint versus 17.3% (23/133) placebo patients (difference of proportion: 55.8 [95% confidence interval: 46.6–65.0]; p<0.0001). Similarly, in each disease aetiology subgroup, more patients met the primary endpoint in the lusutrombopag versus placebo arm (Figure 1).
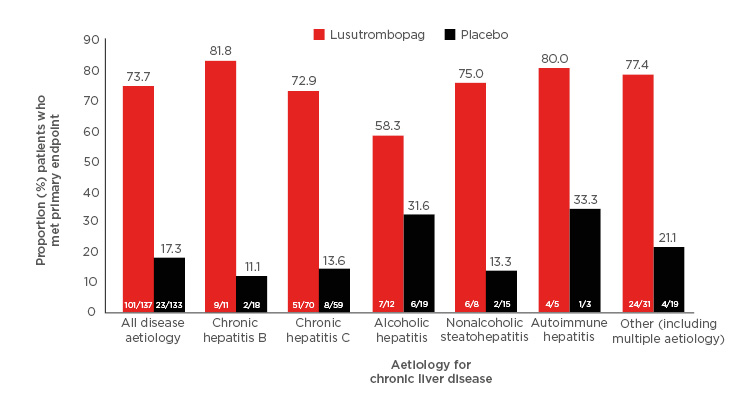
Figure 1: Proportion of patients who met primary endpoint by underlying aetiology for chronic liver disease.
Proportion of patients experiencing ≥1 TEAE were 61.9% for lusutrombopag and 64.5% for placebo; 6.5% and 9.0% of events, respectively, were deemed to be treatment-related. TEAE were generally similar between the two treatment arms across the underlying aetiologies. Thrombosis and thromboembolism-related TEAE occurred in 1.9% (3/155) of patients in the lusutrombopag arm (all deemed serious adverse events) and in 1.9% (3/155) of patients in the placebo arm (all deemed non-serious).
CONCLUSION
Regardless of underlying disease aetiology, lusutrombopag was found to be efficacious compared to placebo in avoiding the need for PT in patients with CLD-TCP scheduled to undergo invasive procedures. Furthermore, TEAE were generally similar between the treatment arms across disease aetiologies.








