Meeting Summary
This symposium was held on the first day of the 2023 International Conference on Malignant Lymphoma (ICML) Congress in Lugano, Switzerland. Björn Chapuy, a Haematologist in the department of Oncology and Tumour Immunology at Benjamin Franklin Campus, Charité – Universitätsmedizin Berlin, Germany, described the rapid pace of development of new treatment options for patients with diffuse large B cell lymphoma (DLBCL) who relapse after their first-line (1L) of therapy, and introduced an expert panel of speakers including both haematologists and a patient representative from the Lymphoma Coalition, Europe.
Philipp Staber, Programme Director for Lymphoma and Chronic Lymphocytic Leukaemia at the Medical University of Vienna, Austria, discussed the importance of tumour boards, and how they are structured, while Natacha Bolaños, Head of membership and alliances for the Lymphoma Coalition Europe, shared insights from a global survey of patients living with DLBCL or relapsed/refractory (R/R) DLBCL. Eva González-Barca, Co-ordinator of the Lymphoma Unit at the Catalan Institute of Oncology, Barcelona, Spain, and Gabriel Brisou, a Haematologist at the Institut Paoli-Calmettes, Marseille, France, presented case studies of patients with R/R DLBCL treated with different therapies at second-line (2L). The panellists also described the supporting data for some of the options for 2L therapy.
The overarching message from the symposium was that involvement of the patient, and potentially their caregiver, in treatment decisions is vital, and that recommendations for treatment should come from a multidisciplinary tumour board composed of pathologists, radiologists, and haemato-oncologists, rather than an individual clinician. Though there is currently no simple answer to which treatment approach should be chosen for each patient, the panel hopes that the next few years will bring a greater understanding of the best choices for individualised therapy.
Introduction
DLBCL accounts for approximately 30% of non-Hodgkin’s lymphomas diagnosed each year.1 Initially, DLBCL is generally treated with rituximab-cyclophosphamide-hydroxydaunomycin-vincristine sulphate-prednisone (R-CHOP) chemotherapy.1 In R/R DLBCL, chimeric antigen-receptor T cell (CAR-T) therapy is often the preferred treatment for patients who are not eligible for autologous stem cell transplant (ASCT). However, there are currently several alternative treatments to consider, including the classical treatment regimens rituximab-gemcitabine-oxaliplatin (R-GemOx) and rituximab-bendamustine (R-Benda), and newer options like tafasitamab-lenalidomide (Tafa-Len) and polatuzumab vedotin-bendamustine-rituximab (Pola-BR). Chapuy stressed that new treatment options are being developed at a rapid pace.
This symposium was specifically designed to address cases of R/R DLBCL that are not CAR-T eligible, in order to identify what might be the right treatment options for different patients, and the rationale used by the speaker behind their treatment decisions. Chapuy emphasised that there are many issues to consider when selecting a 2L therapy in the clinic, including local availability (approval and/or reimbursement policy), treatment sequence, and efficacy in the real-world versus clinical trials.
Shared Decision-Making: When First-Line Fails
Philipp Staber and Natacha Bolaños
Staber explained that the outlook for patients with lymphoma is quite optimistic. Patients with DLBCL who remain event free 2 years after diagnosis can now expect a normal lifespan.2 However, approximately one-quarter of patients relapse during these 2 years and have much worse outcomes, with a median overall survival (mOS) rate estimated at just 7.2 months after progression.2,3
The available treatment options for patients relapsing after 1L DLBCL treatment are growing. There are multiple targets for therapy, particularly on the surface of lymphoma cells, such as cluster of differentiation (CD) 19, CD20, and CD79B (Figure 1).4
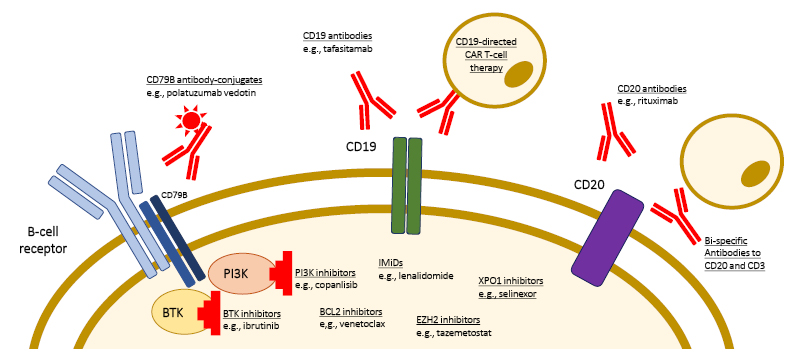
Figure 1: Targets of novel therapeutic approaches for the treatment of patients with relapsed/refractory diffuse large B cell lymphoma.
Adapted from Frontzek et al.4
BTK: Bruton tyrosine kinase; BCL2: B-cell lymphoma 2; CD: cluster of differentiation; EZH2: enhancer of zeste homolog 2; IMID: immunomodulatory imide drug; PI3K: phosphoinositide 3 kinase.
Chimeric Antigen Receptor-T CellsThree CAR-T products have been approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for lymphoma in the third-line (3L) setting: axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel.6 Axicabtagene ciloleucel has recently been approved for use in 2L DLBCL based on the results of the ZUMA-7 clinical trial,7 which demonstrated a considerable advantage over standard of care (high-dose chemotherapy followed by ASCT) with a 2-year event-free survival rate of 41% versus 16%, respectively, and a complete response (CR) rate of 65% versus 32%.8 Staber explained that another option for R/R DLBCL treatment, which has demonstrated impressive results, is Tafa-Len, and data for this therapy is described in further detail in the second part of this article. |
One of the treatments of particular interest currently is CAR-T, a therapy that consists of genetically modified autologous T cells harvested from the patient, bioengineered to express the single chain variable domain of an anti-CD19 antibody coupled to the intracellular T-cell signalling domain of the T-cell receptor. This causes the modified cytotoxic T cells to target cells expressing CD19.5
Tumour Boards for the Management of Patients with Relapsed/Refractory Diffuse Large B Cell Lymphoma
Based on the various treatment options that are currently available, Staber explained that tumour boards are becoming standard practice across Europe and worldwide. The format for a tumour board in Staber’s institute begins with the case manager introducing the individual patient’s situation, including the course of disease, treatment compliance, comorbidities, and the socioeconomic background and expectations of the patient. The board has input from pathologists and laboratory medicine in terms of the diagnosis and risk factors, radiologists in terms of disease extent, and at least two haemato-oncologists who are not directly treating the patient, in terms of treatment options and recommendations. Finally, the case manager discusses the recommended options with the patient. Staber emphasised that therapy recommendations presented to the patient should reflect not just what the data indicates, but also what treatments are locally available, and the preferences of the patient.
A multidisciplinary lymphoma board approach to treatment decisions has a meaningful impact on patients, enhances interaction between disciplines, and furthers education at multiple levels of a cancer care team.9
Insights from a Global Survey of Patients Living with Diffuse Large B-Cell Lymphoma
As a patient representative, Bolaños was ideally placed to present data from a global patient survey conducted by the Lymphoma Coalition. The Lymphoma Coalition is a global network of 80 member organisations, from over 50 countries, that provide support to patients with lymphoma.10 The organisation enables lymphoma patient organisations to share resources, best practices, and policies and procedures, with a view towards achieving equity in lymphoma outcomes across borders.10,11
The 2022 Global Patient Survey on Lymphomas and Chronic Lymphocytic Leukaemia (CLL) included 7,113 responses from patients and 1,524 from caregivers.12 Of all the patients responding, 959 had DLBCL, and 204 had R/R DLBCL (2022 Lymphoma Coalition Global Patient Survey, unpublished data).
Patients with DLBCL or R/R DLBCL felt that the most important features of any new DLBCL treatment were its curative value (77%), improved or longer survival (63%), and improved quality of life (59%). The most frequent psychosocial issues for patients were fear of relapse (63%), fear of progression (37%), anxiety (33%), and depression (25%). Bolaños emphasised that fear of relapse has been consistently associated with significantly worsened general health status, physical, emotional, and social functioning.12 However, Bolaños stressed that only a quarter of patients reported that they had discussed this fear with their clinicians.
In terms of communication between doctors and patients, 43% of patients with DLBCL reported that they were not given more than one treatment option before their current/last therapy. The most important factors in communication (rated as ‘very important’) were considered to be telling the full truth about the diagnosis, even though it may be uncomfortable or unpleasant (74%); understanding the patient’s goals and concerns regarding care options (59%); and asking about the patient’s preferences regarding level of information and involvement in care and decision-making (55%). Bolaños also mentioned that approximately 35% of these patients reported that they would like their caregiver to take part in treatment discussions.
Bolaños concluded that achieving the outcomes that matter to patients is the critical factor in achieving high-quality cancer care. To patients, this is not limited to the clinical outcomes, but is affected by their individual goals and values.
Available Treatments in Second-Line Diffuse Large B Cell Lymphoma Today: Clinical Case Discussions
Eva González-Barca and Gabriel Brisou
Patient Case 1
González-Barca presented the first patient case: a female patient in their early 70s with DLBCL (no MYC rearrangement), who relapsed 24 months after 1L therapy with R-CHOP (Figure 2).
R-GemOxA Phase II trial with R-GemOx in patients with R/R DLBCL (N=32; median age: 69 years) reported an objective response rate (ORR) of 43%, CR rate of 34%, and mOS of 9.1 months (median follow-up [mFU]: 13 months).13 A second Phase II trial in patients with R/R DLBCL (N=49; median age: 69 years) reported similar results, with an ORR of 46%, CR rate of 38%, median progression-free survival (mPFS) of 5 months, and mOS of 11 months (mFU: 65 months).14 A retrospective observational study of R-GemOx in patients with R/R DLBCL who were ASCT-ineligible (N=196; median age: 72 years) reported an ORR of 38%, CR of 33%, mPFS of 5 months, and mOS of 10 months (mFU: 22 months).15 |
Rituximab-bendamustineThe first Phase II R-Benda trial in R/R DLBCL (N=59; median age: 67 years) reported an ORR of 63%, CR rate of 37%, and a mPFS of 6.7 months (mFU: 4.7 months).16 However, González-Barca noted that the trial population was not representative of a European population, as all participants were of Japanese or Korean ethnicity.16 A subsequent Phase II trial in the USA (N=59; median age: 74 years) reported a lower ORR of 46%, CR of 15%, and a mPFS of 3.6 months (mFU: not reported).17 A retrospective, observational study of R-Benda in R/R DLBCL in Italy (N=55; median age: 76 years) reported an ORR of 50%, CR of 28%, and mPFS of 8.8 months (last follow-up: 12–71 months).18 In this first patient case, R-GemOx was selected as the 2L therapy, which resulted in remission after six cycles. However, the patient’s disease relapsed for the second time, 13 months after treatment. |
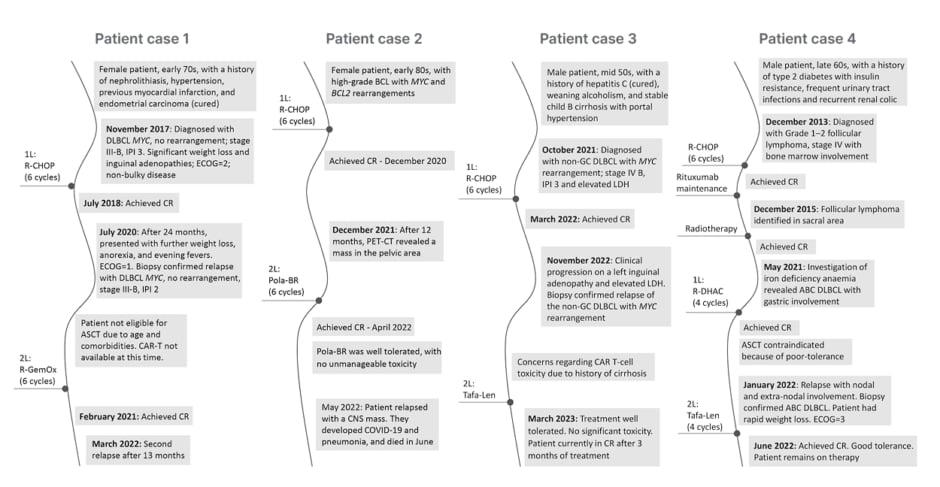
Figure 2: Patient cases illustrating second-line therapy in diffuse large B cell lymphoma.
ABC: activated B cell; ASCT: autologous stem cell transplant; BCL: B-cell lymphoma; CAR-T: chimeric
antigen receptor T-cell therapy; CNS: central nervous system; CR: complete response; DLBCL: diffuse large B-cell lymphoma; ECOG: European Cooperative Oncology Group Performance Status; GC: germinal centre; IPI: International Prognostic Index; LDH: lactate dehydrogenase; Pola-BR: polatuzumab vedotin-bendamustine-rituximab; R-CHOP: rituximab-cyclophosphamide-hydroxydaunomycin-vincristine sulphate-prednisone; R-DHAC: rituximab-dexamethasone-cytarabine-carboplatin; R-GemOx: rituximab-gemcitabine-oxaliplatin; Tafa-Len: tafasitamab-lenalidomide; 1L: first-line; 2L: second-line.
Polatuzumab vedotin-bendamustine-rituximabPolatuzumab, an anti-CD79b monoclonal antibody conjugated to a microtubule-inhibitor, in combination with bendamustine and rituximab (as Pola-BR) was approved in 2020 for use in patients with R/R DLBCL not eligible for ASCT.19 A Phase II trial of Pola-BR compared 40 patients treated with R-Benda with 40 patients treated with Pola-BR (median age: 71 and 67, respectively).20 Compared with patients treated with R-Benda, those treated with Pola-BR had a higher CR rate (40% versus 18%, respectively; p=0.026), mPFS (9.5 months versus 3.7 months; p<0.001) and mOS (12.4 months versus 4.7 months; p=0.002; mFU: 22.3 months).20 Following a single-arm extension of this trial, an exploratory analysis of pooled data for Pola-BR from the safety run-in, randomised, and extension periods was performed (N=152).21 Results indicated that patients receiving Pola-BR at 2L (n=50) experienced a substantial benefit from Pola-BR, with a CR rate of 74%, mPFS of 11.5 months, and mOS of 18.4 months.21 |
The patient was not eligible for ASCT due to age and comorbidities, and González-Barca explained that if CAR-T therapy had been available to the patient at this time, they would have been ineligible because they progressed >12 months after 1L therapy. When this patient relapsed in 2020, available treatment options included R-GemOx and R-Benda. González-Barca stressed that clinical trial data is limited in this population; few trials have been conducted, and these included only a small number of patients.
Patient Case 2
The second patient case presented by González-Barca was a female in their early 80s with high-grade B cell lymphoma, with IgMYC and IgBCL2 rearrangements, who relapsed 12 months after 1L therapy with R-CHOP, with a mass in the pelvic area (Figure 2). The patient was treated with Pola-BR at 2L, and they achieved CR after four months. Despite responding well, with no unmanageable toxicity, the patient had a second relapse with central nervous system involvement approximately 1 month later, and died after contracting COVID-19 and developing pneumonia.
González-Barca concluded from these first two cases that the treatment of patients with R/R DLBCL not eligible for transplant remains an unmet clinical need. Although CAR-T therapy offers hope in this environment, it is not available in all medical centres, nor to all patients.
Patient Case 3
The third patient case was presented by Brisou: a male patient in their mid-50s with Stage IVB, International Prognostic Index (IPI) 3, non-germinal centre DLBCL, with MYC rearrangement, elevated lactate dehydrogenase, and a history of cirrhosis. Thirteen months after 1L therapy with R-CHOP, the patient relapsed with clinical progression on a left inguinal adenopathy and elevated lactate dehydrogenase (Figure 2).
This patient was treated at 2L with Tafa-Len due to concerns regarding CAR-T therapy in the context of the cirrhosis. Tafa-Len was well tolerated, and CR was achieved after 3 months of treatment.
Patient Case 4
The final patient case presented was that of a male patient in their late 60s who developed activated B cell DLBCL with gastric involvement, following a history of Grade 1–2 follicular lymphoma 8 years earlier (treated with R-CHOP and radiotherapy). The patient achieved CR after two cycles of rituximab-dexamethasone-cytarabine-carboplatin, and ASCT was contraindicated because of poor tolerance. The patient was treated with a further two cycles of rituximab-dexamethasone-cytarabine-carboplatin with persistent CR. Six months later, the patient relapsed with nodal and extra-nodal involvement, and was in poor condition, with B symptoms, rapid weight loss, and European Cooperative Oncology Group Performance Status (ECOG-PS) of 3 (Figure 2).
The patient was treated with Tafa-Len at 2L, and CR was achieved after four cycles. At the time of presentation (June 2022), the patient still presented good tolerance to treatment and remained on therapy.
Brisou emphasised that these last two patient cases illustrate that in the real world, patients may be ineligible for CAR-T therapy for reasons other than age, such as comorbidities or rapidly progressing disease. Brisou also highlighted that in both cases, the patients attained a durable CR without significant toxicity with Tafa-Len, despite large tumour burdens.
Tafa-Len has been available in France via an early access programme for 1.5 years. Brisou described encouraging patient outcomes in this programme at the Institut Paoli Calmettes in Marseille (N=22, median age: 74 years), with an ORR of 50% at 2L (58% at 3L) and CR rate of 50% (25% at 3L). Most patients who achieved CR had durable responses and were still receiving treatment. Brisou concluded that Tafa-Len is a well-tolerated regimen suitable for administration through an outpatient clinic. The best patient profile for Tafa-Len therapy, in Brisou’s opinion, is a patient with one prior line of therapy, without primary-refractory disease, who is not eligible for CAR-T therapy.
Tafasitamab-lenalidomideTafasitamab is an anti-CD19 monoclonal antibody approved in 2021 for use in combination with lenalidomide in patients with R/R DLBCL ineligible for ASCT.22 The pivotal study for Tafa-Len was L-MIND, a Phase II trial in patients with R/R DLBCL not eligible for high-dose chemotherapy or ASCT (n=80; median age: 72 years).23-25 Brisou emphasised that this study excluded patients with more advanced disease.24 Half of the patients in this trial were in 2L. At 5-year follow-up, the overall population had an ORR of 58% and CR rate of 41%.26 Responses were durable, with a median duration of response that was not reached.26 Responses in patients receiving Tafa-Len at 2L (n=40; median age: 72 years) were an ORR of 68%, CR of 53%, mPFS of 23.5 months, and the mOS was not reached.26 The RE-MIND2 study retrospectively compared L-MIND data with patient-level matched, observational cohorts of DLBCL patients (N=3,454), showing that Tafa-Len was associated with significantly better mOS compared with other systemic therapies pooled (34.1 months versus 11.6 months; p=0.0068), R-GemOx (31.6 months versus 11.0 months; p=0.0003), Pola-BR (20.1 months versus 7.2 months; p=0.034), and rituximab-lenalidomide (24.6 months versus 7.4 months; p=0.012).27,28 |
Panel Discussion with Questions from the Audience
Eva González-Barca, Natacha Bolaños, Björn Chapuy, Gabriel Brisou, and Philipp Staber
What is the Role of Caregivers in the Decision-Making Process for Diffuse Large B Cell Lymphoma Therapy?
This depends on the preferences of the patient. In Bolaños’ experience, some patients prefer the caregiver to discuss treatment options with the clinician on their behalf, while other patients may want their caregivers to be involved because of the active role their caregiver will need to take in accessing treatment.
Approximately Half of the Patients in L-MIND Experienced Neutropenia, Possibly Because of the Lenalidomide. What is the Ideal Combination Dose for Lenalidomide with Tafasitamab?
In clinical practice, Brisou explained that they tend to start patients on 25 mg of lenalidomide per day, and if mild neutropenia occurs it is managed with granulocyte colony-stimulating factor. Brisou sometimes lowers the dose for older patients after the first cycle if they do not tolerate it well.
What Kind of Kinetics of Response Should be Expected with Tafasitamab-Lenalidomide? If the Duration of Response is Generally Good, How Long Should Clinicians Wait Before Considering Stopping Therapy?
Brisou emphasised that, in their clinic, when the treatment works, it is usually apparent by 1 month of treatment. If patients have not responded by that time, Brisou feels that it is unlikely that they will do so.
Can Tafasitamab-Lenalidomidebe Used After Failure to Respond to Polatuzumab Vedotin-Bendamustine-Rituximab?
Brisou explained that bendamustine kills T cells, whereas lenalidomide activates them. For this reason, Brisou feels that they would be unlikely to use this sequence of regimens. Chapuy suggested that this might be possible if the wash-out period between the two therapies is long enough for the T cell compartment to be reinvigorated.
Are There Any Data on Differential Responses to Tafasitamab-Lenalidomide According to Cell of Origin? This Might Help with Treatment Decisions for Individual Patients.
The faculty accepted that there is a lack of good biomarkers to predict responses to different therapies at this point. González-Barca would be more likely to choose between therapy options on a clinical rather than a biological basis; for example, using Pola-BR in patients with a better level of health, since bendamustine is immunosuppressive; and using Tafa-Len in older patients and those with comorbidities, since it is well tolerated.
Could Bendamustine Be Removed from the Polatuzumab Vedotin-Bendamustine-Rituximab Regimen, Because It May Decrease the Response Rate to Tafasitamab-Lenalidomide and Bi-specific Antibodies at a Subsequent Relapse?
Chapuy explained that it can be difficult to change a combination therapy from that used in the trial which led to its approval. However, in practice, they feel that many clinicians do reduce the bendamustine component of the therapy down to almost zero; for example, prior to CAR-T therapy. Staber agreed that these considerations come down to the clinician and what they are planning for the future treatment of a patient.
Closing Remarks
Björn Chapuy
Chapuy stressed that it is important that patients and their clinicians reach an agreement on the goal of treatment. They explained that one early decision to make is whether a curative or non-curative approach will be used. Once this decision is made, consideration of the locally available treatments can guide treatment recommendations. Chapuy reiterated that decisions about which treatments to recommend to a patient should come from a multidisciplinary tumour board, rather than an individual clinician.
In terms of the choice of therapy for DLBCL at 2L, the take-home message from this symposium was that there is currently no simple answer. The Chair, Chapuy, concluded that the next few years will bring a greater understanding of the best choices for individual patients. Chapuy concluded that although there is much to do in this field, they are astounded by the pace of development of new agents and combinations over the past 2–3 years. Some of these new treatments can be curative even in a patient over 80 years of age. However, something that patients and clinicians alike must face is the cost of therapy, and varying rules for reimbursement in different countries.






